Question
Question: When phenol is treated with \({{D}_{2}}S{{O}_{4}}/{{D}_{2}}O\) some of the hydrogen atoms get exchan...
When phenol is treated with D2SO4/D2O some of the hydrogen atoms get exchanged. The final product in this exchange reaction is:
A. 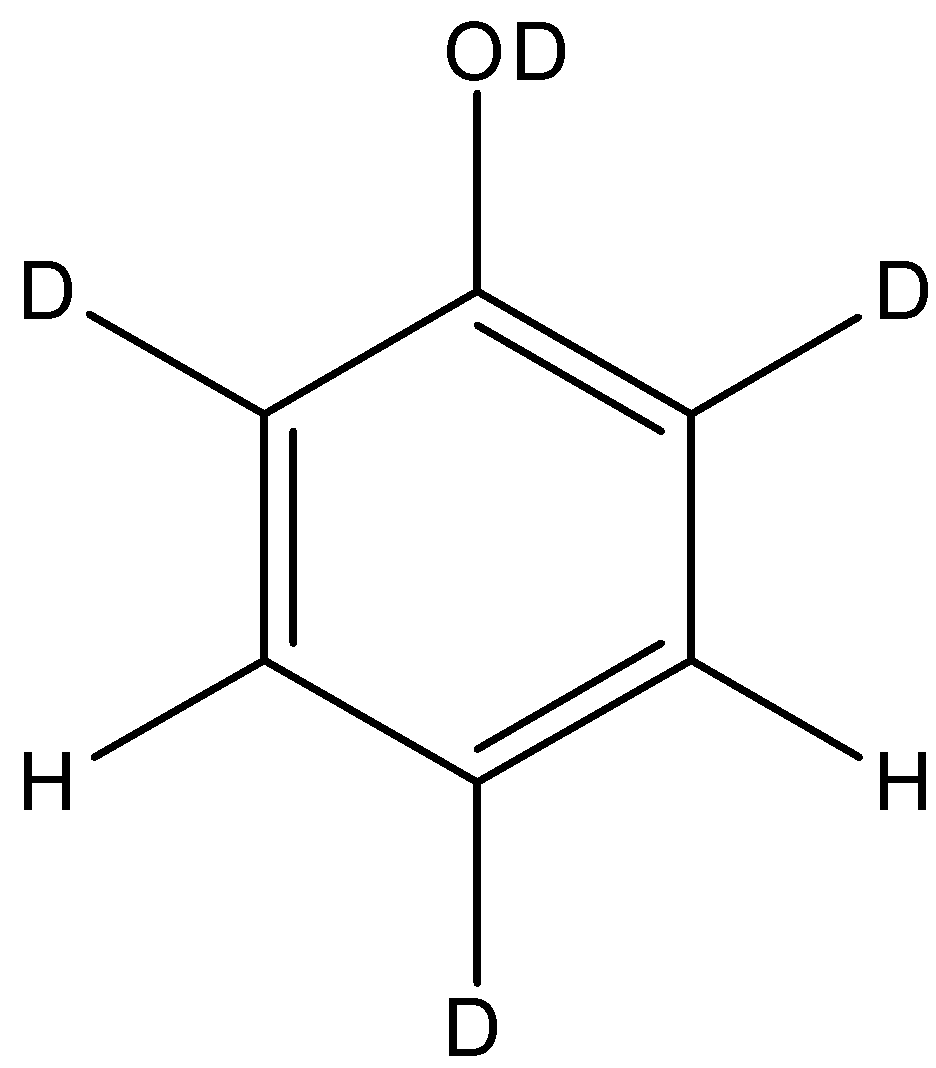
B. 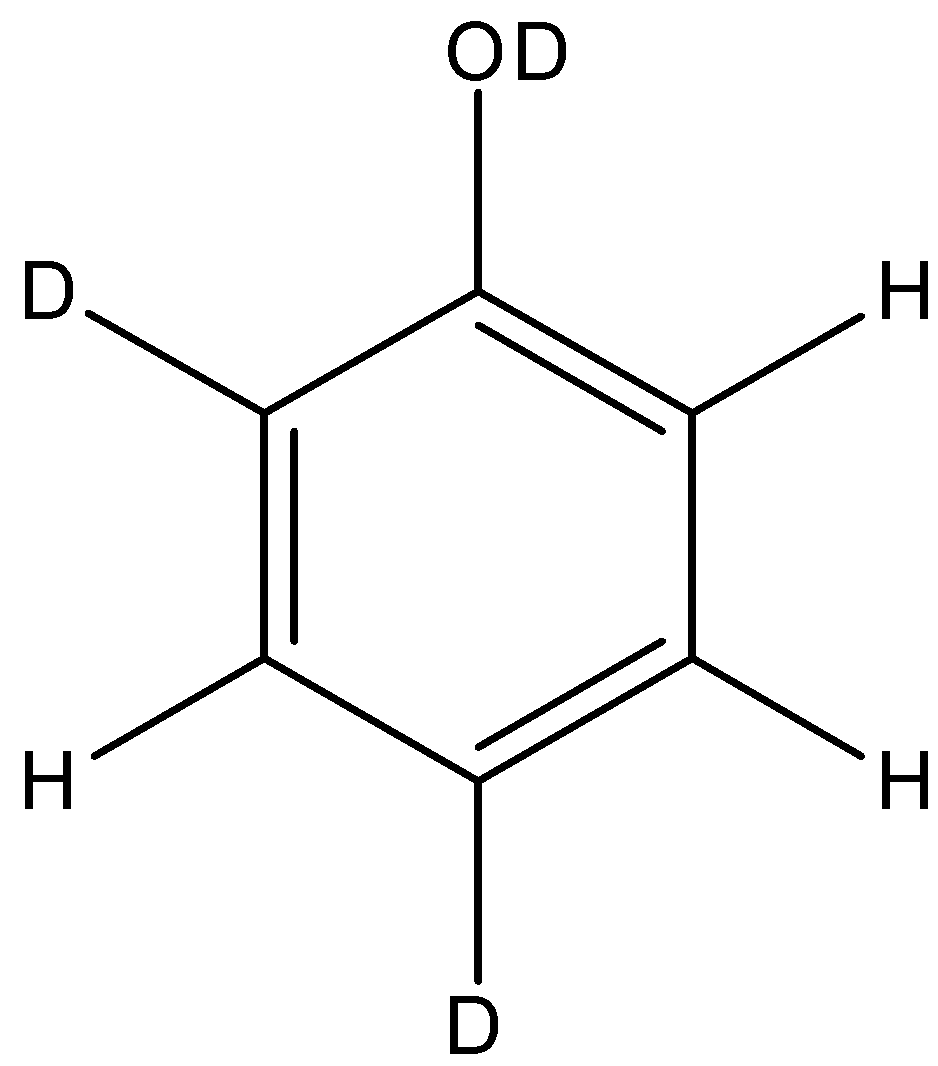
C. 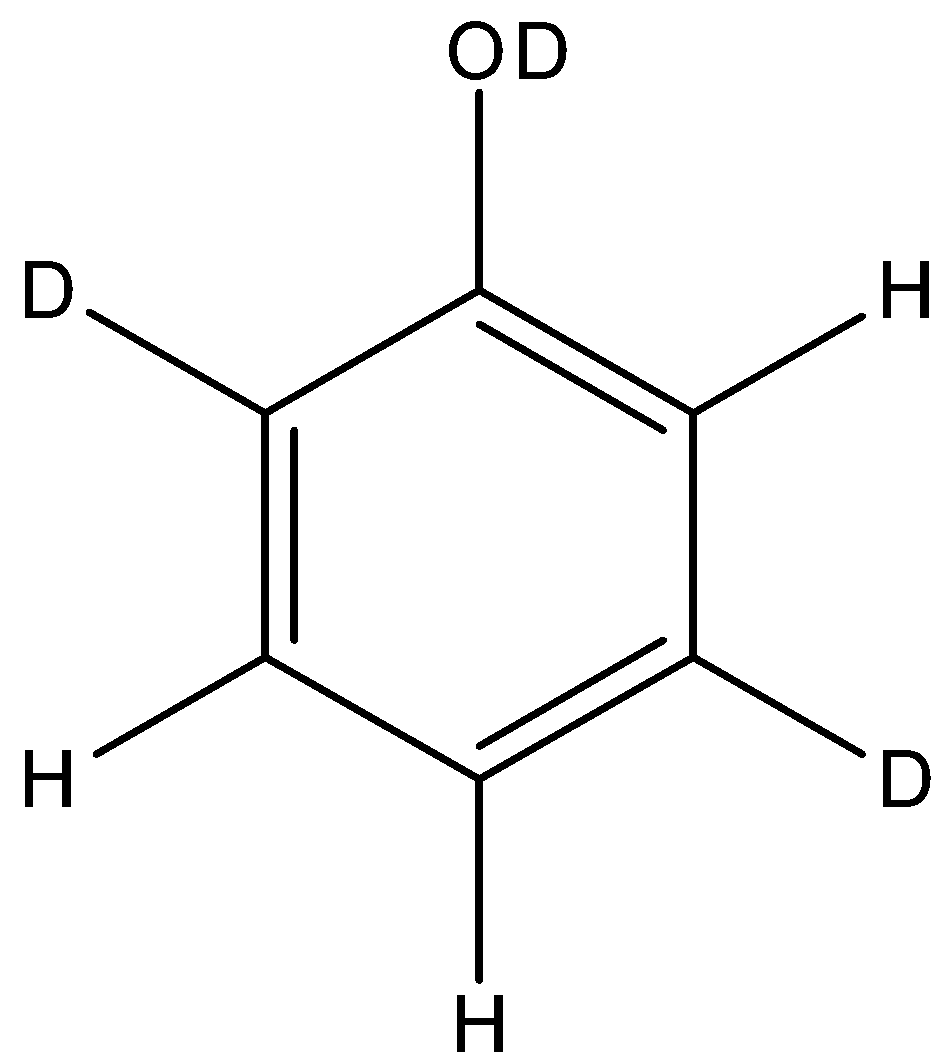
D. 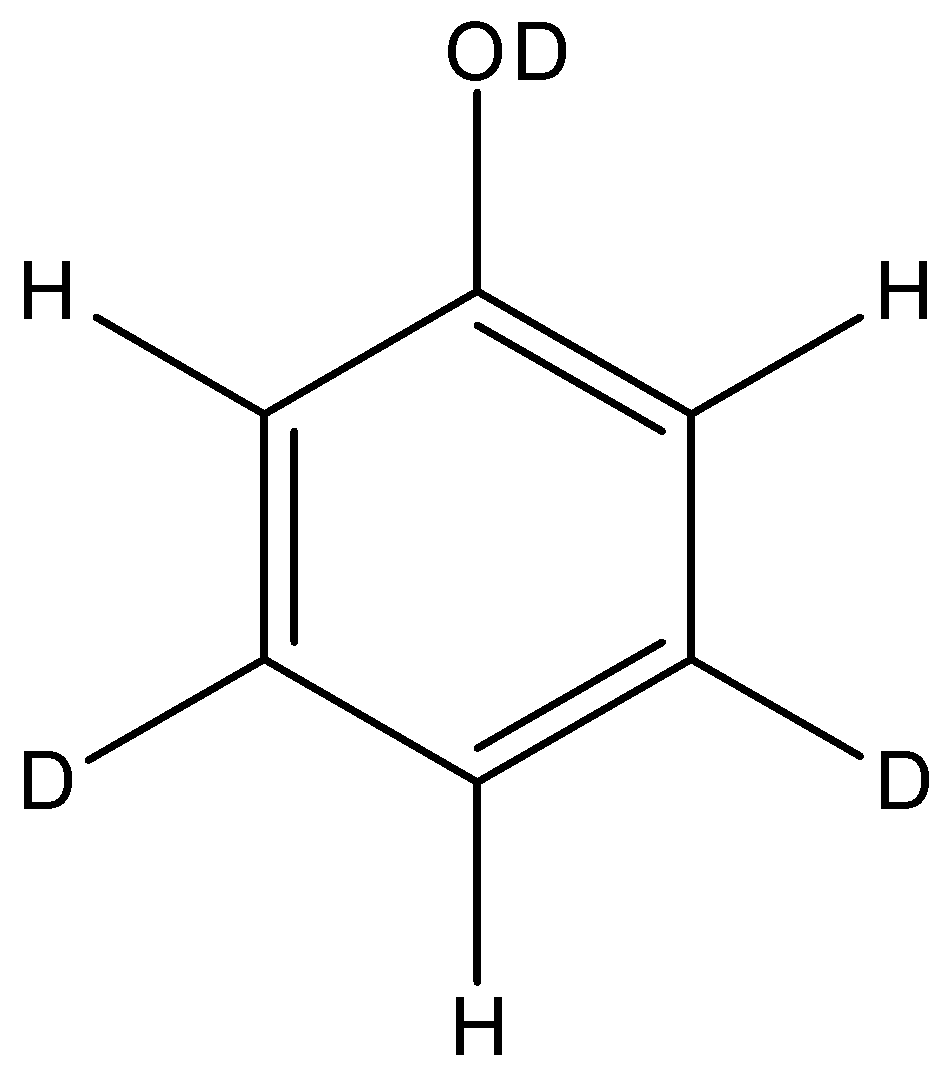
Solution
Think about the conditions in which replacement is likely to occur, will the sites of replacement be electron rich or electron deficient? Consider the resonating structures of phenol that are possible in acidic medium.
Complete step by step answer:
Phenol is present in the acidic medium of D2SO4/D2O.We know that the hydroxyl group is an electron donating group on the benzene ring since the oxygen atom has multiple lone pairs which can be used to stabilize the ring. The resonance structures are as follows:
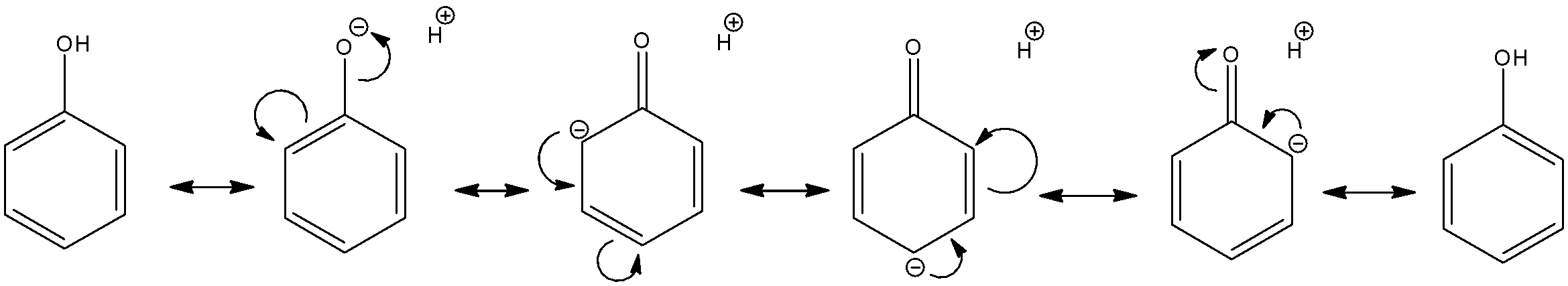
As we can see here, the hydrogen atom on OH− moiety is an acidic proton, thus, it will definitely get exchanged during the reaction. So, the OH− functional group will become a OD− functional group.
We also see that the ortho- as well as para- sites have an electron rich nature. The electron donation oxygen causes electrons to be concentrated at the 2,4,6 sites. Thus, the electrophilic groups like H+ or D+ will be attracted to these sites. Since, the medium present is D2SO4/D2O, these sites will exchange hydrogen for deuterium. So, the final product will have deuterium in the OD− group as well as all the ortho- and para- sites.
So, the final structure of the phenol after the reaction will be:
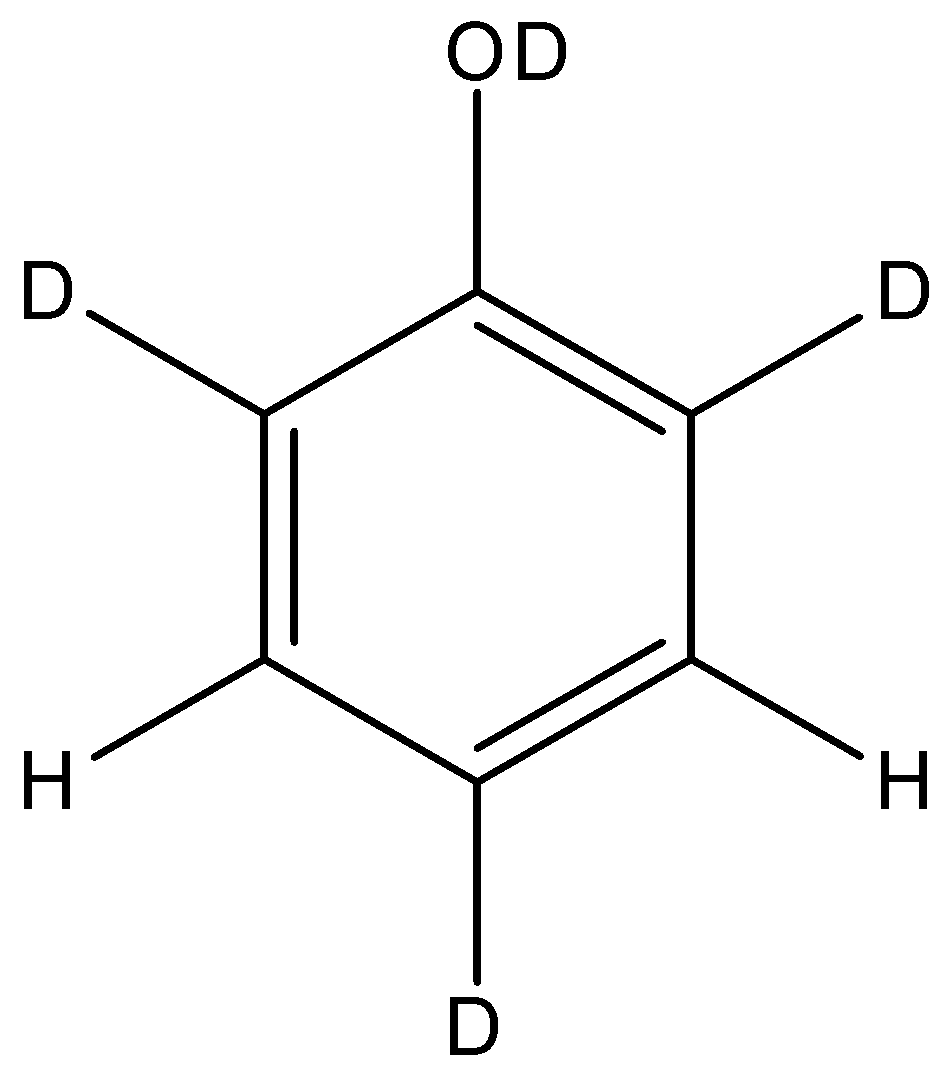
So, the correct answer is “Option A”.
Note: It may seem like the hydroxyl group will be an electron withdrawing group due to the fact that oxygen is a highly electronegative atom. But take into consideration the fact that oxygen is an element from the second period and thus has no vacant d-orbitals to accept electrons after its valency has been satisfied. So after it has formed 2 bonds, it will act as an electron donating group.
