Question
Question: When N₂(g) and H₂(g) are mixed N₂H₄ (g), NH₃(g) or both may form, depending upon the relative amount...
When N₂(g) and H₂(g) are mixed N₂H₄ (g), NH₃(g) or both may form, depending upon the relative amount of N₂ and H₂ taken. If initial moles of N₂, H₂ are x, y and final moles of N₂H₄, NH₃ are z, v, then the correct options from the following in order of (x, y, z, v) is/are -
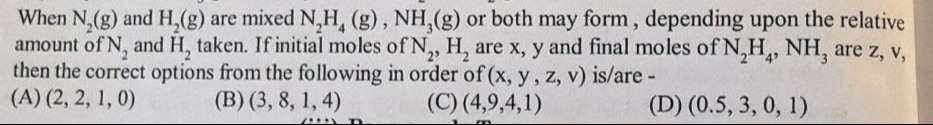
(2, 2, 1, 0)
(3, 8, 1, 4)
(4,9,4,1)
(0.5, 3, 0, 1)
(A), (B), (D)
Solution
The reactions that can occur are:
- N₂(g) + 2H₂(g) → N₂H₄(g)
- N₂(g) + 3H₂(g) → 2NH₃(g)
Let the initial moles of N₂ be x and H₂ be y. Let the final moles of N₂H₄ be z and NH₃ be v.
Let the moles of N₂ consumed to form N₂H₄ be z.
Let the moles of H₂ consumed to form N₂H₄ be 2z.
Let the moles of N₂ consumed to form NH₃ be v/2.
Let the moles of H₂ consumed to form NH₃ be 3v/2.
The total moles of N₂ consumed = z+v/2.
The total moles of H₂ consumed = 2z+3v/2.
For the final state to be reachable from the initial state, the amount of reactant consumed cannot exceed the initial amount of the reactant. So, we must have:
Initial moles of N₂ ≥ Moles of N₂ consumed ⟹x≥z+v/2
Initial moles of H₂ ≥ Moles of H₂ consumed ⟹y≥2z+3v/2
We also need to check the conservation of atoms.
Initial number of N atoms = 2x
Initial number of H atoms = 2y
Final number of N atoms = 2×(final moles of N₂H₄)+1×(final moles of NH₃)+2×(final moles of N₂)
Final number of H atoms = 4×(final moles of N₂H₄)+3×(final moles of NH₃)+2×(final moles of H₂)
Let the final moles of N₂ be xf and H₂ be yf.
xf=x−(z+v/2)
yf=y−(2z+3v/2)
Since the final amounts cannot be negative, xf≥0 and yf≥0. These are the same as the conditions derived from reactant consumption.
Let's check each option:
(A) (x, y, z, v) = (2, 2, 1, 0)
x=2,y=2,z=1,v=0
Check conditions:
x≥z+v/2⟹2≥1+0/2⟹2≥1 (True)
y≥2z+3v/2⟹2≥2(1)+3(0)/2⟹2≥2+0⟹2≥2 (True)
Moles of N₂ consumed = 1+0/2=1. Remaining N₂ = 2−1=1.
Moles of H₂ consumed = 2(1)+3(0)/2=2. Remaining H₂ = 2−2=0.
Final state: 1 mole N₂H₄, 0 moles NH₃, 1 mole N₂, 0 moles H₂.
Initial N atoms = 2(2)=4. Final N atoms = 2(1)+1(0)+2(1)=4. (Conserved)
Initial H atoms = 2(2)=4. Final H atoms = 4(1)+3(0)+2(0)=4. (Conserved)
Option (A) is consistent.
(B) (x, y, z, v) = (3, 8, 1, 4)
x=3,y=8,z=1,v=4
Check conditions:
x≥z+v/2⟹3≥1+4/2⟹3≥1+2⟹3≥3 (True)
y≥2z+3v/2⟹8≥2(1)+3(4)/2⟹8≥2+6⟹8≥8 (True)
Moles of N₂ consumed = 1+4/2=3. Remaining N₂ = 3−3=0.
Moles of H₂ consumed = 2(1)+3(4)/2=2+6=8. Remaining H₂ = 8−8=0.
Final state: 1 mole N₂H₄, 4 moles NH₃, 0 moles N₂, 0 moles H₂.
Initial N atoms = 2(3)=6. Final N atoms = 2(1)+1(4)+2(0)=2+4=6. (Conserved)
Initial H atoms = 2(8)=16. Final H atoms = 4(1)+3(4)+2(0)=4+12=16. (Conserved)
Option (B) is consistent.
(C) (x, y, z, v) = (4, 9, 4, 1)
x=4,y=9,z=4,v=1
Check conditions:
x≥z+v/2⟹4≥4+1/2⟹4≥4.5 (False)
Option (C) is inconsistent because it requires more N₂ than initially available.
(D) (x, y, z, v) = (0.5, 3, 0, 1)
x=0.5,y=3,z=0,v=1
Check conditions:
x≥z+v/2⟹0.5≥0+1/2⟹0.5≥0.5 (True)
y≥2z+3v/2⟹3≥2(0)+3(1)/2⟹3≥0+1.5⟹3≥1.5 (True)
Moles of N₂ consumed = 0+1/2=0.5. Remaining N₂ = 0.5−0.5=0.
Moles of H₂ consumed = 2(0)+3(1)/2=1.5. Remaining H₂ = 3−1.5=1.5.
Final state: 0 moles N₂H₄, 1 mole NH₃, 0 moles N₂, 1.5 moles H₂.
Initial N atoms = 2(0.5)=1. Final N atoms = 2(0)+1(1)+2(0)=1. (Conserved)
Initial H atoms = 2(3)=6. Final H atoms = 4(0)+3(1)+2(1.5)=0+3+3=6. (Conserved)
Option (D) is consistent.
All options (A), (B), and (D) are consistent with the conservation of mass and the stoichiometry of the reactions.
