Question
Question: When methane is heated with oxygen in the presence of \( M{o_2}{O_3} \) catalyst, the organic produc...
When methane is heated with oxygen in the presence of Mo2O3 catalyst, the organic product obtained is:
A: methanal
B: ethanol acid
C: methanol
D: ethanol
E: 2−methylpropan−2−ol
Solution
Hint : As we know that Methane is basically a highly stable compound. Thus, a high amount of activation energy is needed to break the C−H bond or to oxidize the molecule. Hence, a higher temperature (usually around 500k ) of catalyst is required for the oxidation of methane in comparison to longer chain hydrocarbons.
Complete Step By Step Answer:
As we know that Heating of methane with oxygen in the presence of Mo2O3 catalysts is an oxidation reaction. so, heating is done in order to activate the catalyst i.e. Molybdenum oxide ( Mo2O3 ). The process is generally known as the catalytic oxidation of methane. We can show the chemical equation for the process as written below:
CH4+O2Mo2O3ΔHCHO+H2O
The mechanism of the reaction can be depicted as follows:
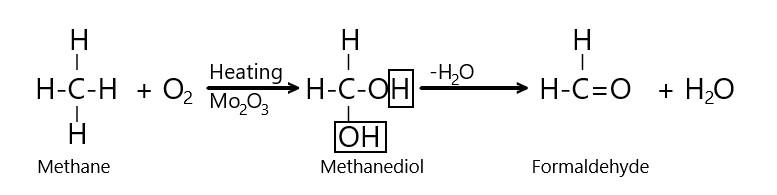
Thus, it is clear from the reaction mechanism that combustion of methane in the presence of Molybdenum oxide results into the production of formaldehyde or methanal (i.e. HCHO ) and water.
Hence, the correct answer is Option A i.e. methanal.
Additional Information:
Similar to the aforementioned reaction, if we replace the catalyst with another one, a different product will be formed. For instance, if a mixture of methane and oxygen is made to react in the presence of copper tubes at a temperature of 523K and 100 atm pressure, methyl alcohol or methanol is produced instead of methanal. The reaction is depicted below:
CH4+O2CuΔCH2OH+H2O
Methanol can also be oxidized to methanal in the presence of oxidizing agents such as acidified potassium dichromate.
Note :
Always remember that when combustion of methane takes place the product which is generally formed is always an aldehyde. Hence, heating of methane results in the formation of Formaldehyde and water.
