Question
Question: When hypophosphorous acid is treated with diazonium salts, it is reduced to: A. arenes B. amines...
When hypophosphorous acid is treated with diazonium salts, it is reduced to:
A. arenes
B. amines
C. ethyl alcohol
D. methane
Solution
We know that the general formula of diazonium salt is RN2+X−. The R represents any aryl group and X− may be chloride ion, bromide ion, HSO4− or BF4−. The naming of diazonium salt is done by using the word ‘diazonium’ as a suffix to the name of the parent hydrocarbon followed by the name of anion.
Complete step by step answer:
The diazonium salts undergo two types of reaction. 1 type is a reaction in which nitrogen is displaced and in another type retention of the diazo group takes place.
Now, come to the question. The reaction of hypophosphorous acid is treated with diazonium salts is a type of reaction in which nitrogen is displaced. Hypophosphorous acid is a mild reducing agent. So, it reduces diazonium salts to arenes and themselves get oxidized to phosphoric acid. Ethanol is also a mild reducing agent. On reacting ethanol with diazonium salt, diazonium salt converted to arenes and ethanol oxidized to ethanol.
ArN2+Cl−+H3PO2+H2O→ArH+N2+H3PO3+HCl
(Ar represents benzene ring)
So, the correct answer is Option A.
Additional Information:
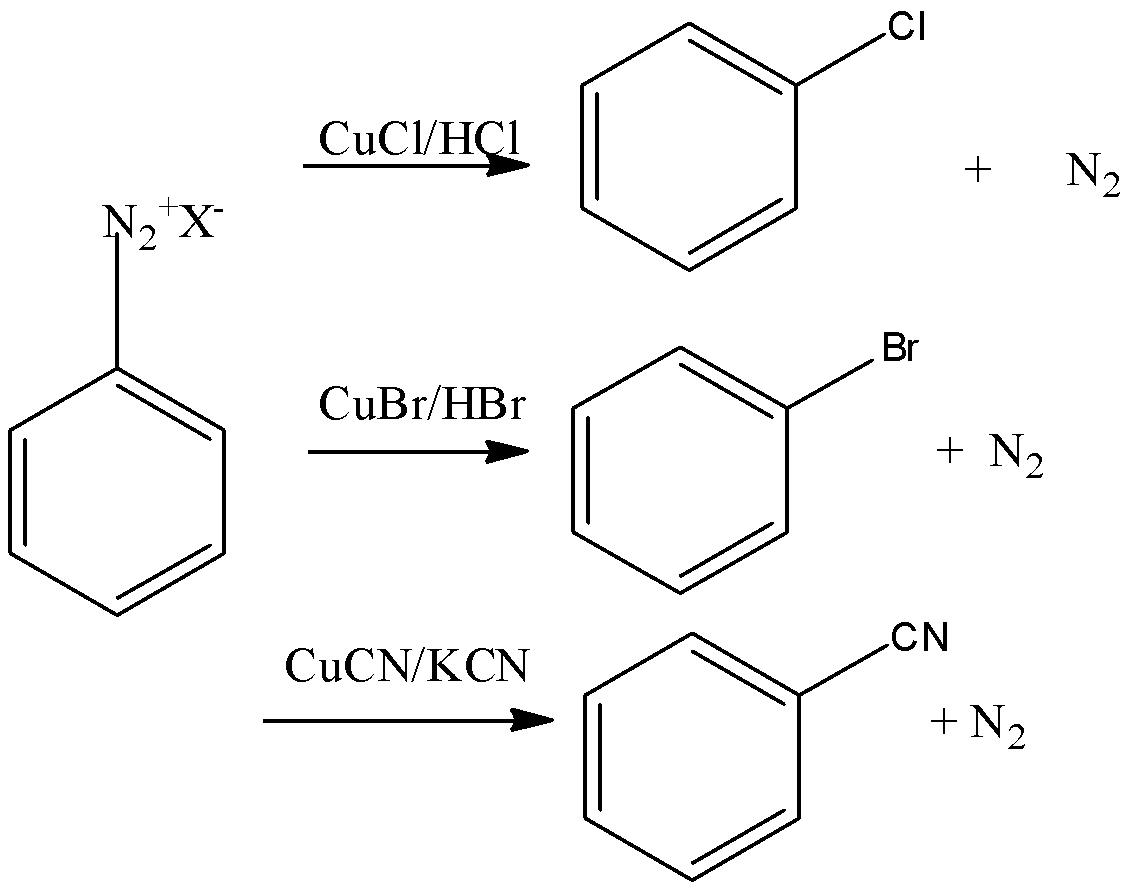
Let’s discuss Gatterman's reaction in brief. In this reaction, the introduction of fluorine, bromide and iodine in the benzene ring is done by reacting diazonium salt solution with corresponding halogen acid in presence of power of copper.
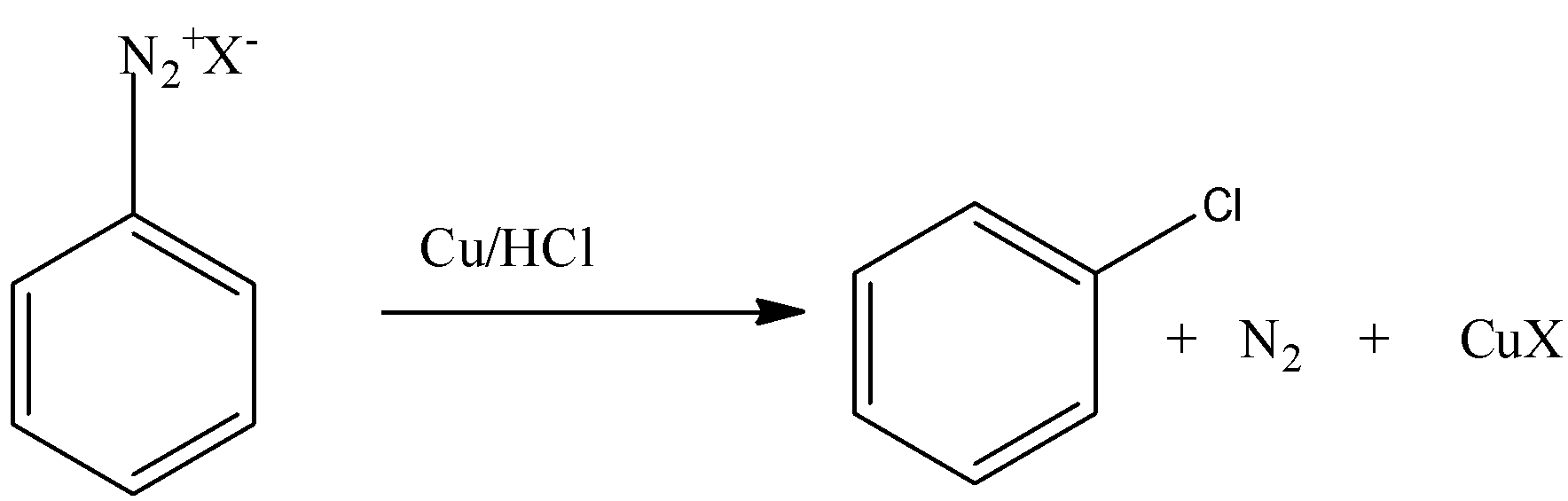
Note: Diazonium group is a very good leaving group. Because of this property the chloride, fluoride, iodide etc. can be substituted easily. Sandmeyer reaction is the reaction in which chloride, bromide, cyanide ions can be introduced to the benzene ring in the presence of copper (I) ion.