Question
Question: When hydrogen peroxide is treated with a cold acidified \[{K_2}C{r_2}{O_7}\] solution containing eth...
When hydrogen peroxide is treated with a cold acidified K2Cr2O7 solution containing ether, blue colour is obtained. This is due to:
A. Perchromic acid
B. Potassium chromate
C. Chromium sulphate
D. Chromium trioxide
Solution
Potassium dichromate or K2Cr2O7 is an orange coloured oxidizing agent, used in various reactions. On the other hand, hydrogen peroxide or H2O2 is a pale blue viscous liquid. They react with each other and give blue colour compounds.
Complete answer:
As we know, potassium dichromate (K2Cr2O7) is orange in colour and on reaction with pale blue hydrogen peroxide, it yields blue colour compound. To identify the blue colour compound we must write the chemical reaction as follows:
K2Cr2O7+4H2O2+H2SO4EtherCrO5+K2SO4+5H2O
The reaction proceeds in three different steps:
Step I: K2Cr2O7+H2SO4→K2SO4+H2Cr2O7
Step II: 4H2O2→4H2O+4(O)
Step III: H2Cr2O7+4(O)→2CrO5+H2O
Hence the three products formed are CrO5, K2SO4 and H2O. Among these, CrO5 or chromium pentoxide has a blue colour in ether.
In the above reaction, the oxidation state of hydrogen peroxide changes from −1 to −2 as it acts as an oxidizing agent in the reaction.
In less acidic conditions, reaction of hydrogen peroxide with potassium dichromate gives a deep blue-violet coloured compound. Whereas, in an alkaline solution this reaction gives a red-brown coloured compound.
Hence the correct option is A, per-chromic acid.
Additional information:
CrO5 or chromium pentoxide is also known as perchromic acid and it has a structure of butterfly.
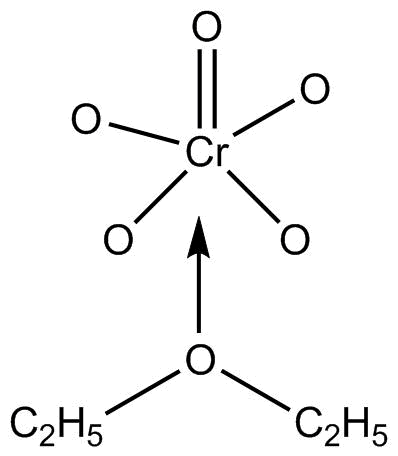
The structure is so, due to two peroxy linkages in the compound.
Note:
Potassium dichromate is an oxidizing agent that oxides aldehydes and alcohols (primary and secondary). It is toxic in nature and also known to be a carcinogen and a mutagen. Therefore, it can be fatal for the environment. It is also a common reason behind dermatitis, especially on arms and hands.
