Question
Question: When \({{{H}}_2}{{{O}}_2}\) is added to ice cold solution of \({{{K}}_2}{{C}}{{{r}}_2}{{{O}}_7}/{{{H...
When H2O2 is added to ice cold solution of K2Cr2O7/H+ in ether, yields blue color solution of oxide of chromium. The oxidation state of chromium is
A. +4
B. +6
C. +8
D. +3
Solution
The given reaction is a redox reaction. H2O2 is added to an acidified solution of potassium dichromate to obtain a redox reaction. Redox reactions involve exchange of electrons from one to another. When the electrons are exchanged completely, then the number of charges on an atom is called oxidation state or oxidation number.
Complete answer:
Potassium dichromate acts as a very strong oxidizing agent which can be used to oxidize primary alcohols, aldehydes etc.
As we know that the potassium dichromate is a very good oxidizing agent and hydrogen peroxide is a very good reducing agent. It is mentioned earlier that the given reaction is a redox reaction. Thus potassium dichromate is reduced and hydrogen peroxide is oxidized.
When H2O2 is added to ice cold solution of K2Cr2O7/H+ in ether, chromium which is in +6 oxidation state is changed to +3 oxidation state. While oxygen in hydrogen peroxide is changed from −1 to 0.
The chemical reactions are given below:
K2Cr2O7+4H2O2+H2SO4→2CrO5+K2SO4+5H2O
4CrO5+6H2SO4→2Cr2(SO4)3+6H2O+7O2
The structure of CrO5 is given below.
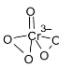
It is known as a butterfly structure. The chemical name of this compound is perchromic acid and it has two peroxy bonds. It forms like a pentagon.
The blue color is due to the formation of this butterfly structured CrO5. In this compound, the oxidation state of chromium is +3.
Hence the correct option is D.
Note:
The blue color occurs very rapidly when the hydrogen peroxide is added to the acidic potassium dichromate solution in the presence of ether. But the blue color fades to a different color, i.e. pale blue-green color which is due to the presence of hexa aqua chromium(III) ions.
