Question
Question: When Grignard reagent reacts with a ketone, it yields: (A) \({{3}^{\circ }}\) alcohol (B) Ethano...
When Grignard reagent reacts with a ketone, it yields:
(A) 3∘ alcohol
(B) Ethanol
(C) 1∘ alcohol
(D) 2∘ alcohol
Solution
The ketone is an organic compound in which there are two alkyl groups. So, when the ketone reacts with Grignard reagent, one more alkyl group attaches to the carbon atom having the double bond and to the same carbon atom alcohol group attaches.
Complete step by step solution:
The Grignard reagent is an organic compound in which an alkyl group, a halogen, and magnesium atom are present. In Grignard reagent, the positive part is the magnesium halide and the negative part is the alkyl group. The Grignard reagent is used to convert aldehydes and ketones to alcohols.
In 1∘ alcohol, the alcohol group is attached to the carbon atom which is further attached to one more carbon atom.
In 2∘ alcohol, the alcohol group is attached to the carbon atom which is further attached to two more carbon atoms.
In 3∘ alcohol, the alcohol group is attached to the carbon atom which is further attached to three more carbon atoms.
So, we know that the ketone has two alkyl groups, and when it reacts with when the ketone reacts with Grignard reagent, one more alkyl group attaches to the carbon atom having the double bond and to the same carbon atom alcohol group attaches. This means that the alcohol group is attached to the carbon atom which is further attached to three carbon atoms. Hence it will form 3∘ alcohol. The reaction is given below:
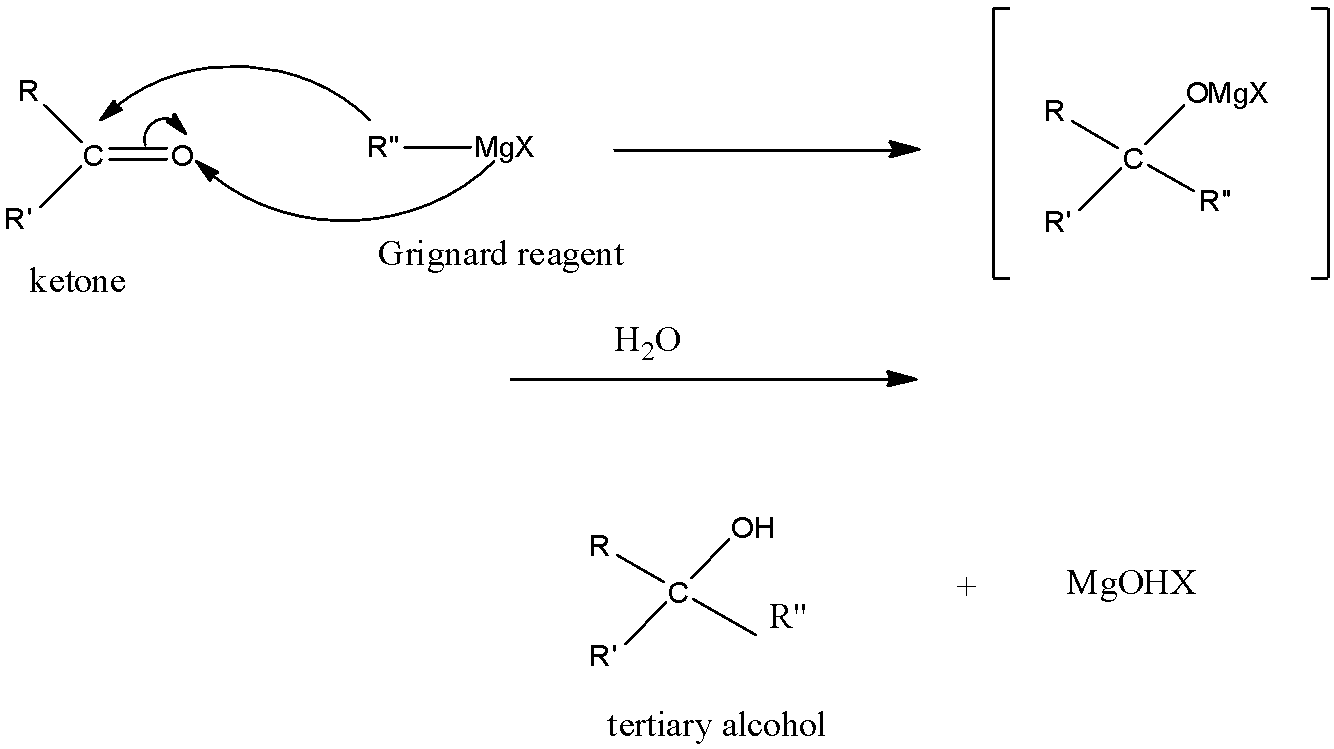
So, the correct answer is an option (A)- 3∘ alcohol.
Note: We can prepare primary alcohol only with formaldehyde. The rest of the aldehyde molecules will always yield secondary alcohol. Ethanol cannot be prepared by this method.
