Question
Question: When glycerol is treated with excess of \(HI\), it produces: A. \[2 - iodopropane\] B. \[allyl{...
When glycerol is treated with excess of HI, it produces:
A. 2−iodopropane
B. allyl iodide
C. propene
D. glycerol triiodide
Solution
Glycerol is also called as Glycerin and is a colorless and an odorless liquid, and is non-toxic in nature and has a sweet taste, its IUPAC name is propane−1,2,3−triol. It has many antiviral and antifungal properties so it is very important for treating wounds and burns.
Complete answer:
Glycerol is also referred to as polyol as it consists of many alcohols. When we talk about its solubility it is soluble in water and is hygroscopic in nature which means it has a tendency to attract the water molecules around it.
Now, when glycerol is treated with an excess of HI, that reactions follow in steps as follows:
In the first step, a molecule of glycerol reacts with three moles of HImolecules to give a product1,2,3−triiodopropane, which is unstable in nature and this formed intermediate loses a molecule of iodine and hence the formation of allyl iodide takes place, then again the formed Allyl iodide adds a molecule of HI to obtain an unstable molecule which loses a molecule of iodine to form propene. Then the formed propene undergoes an addition reaction and HI molecule is added to the propene to give 2−iodopropane. This can be explained by the reaction as follows:
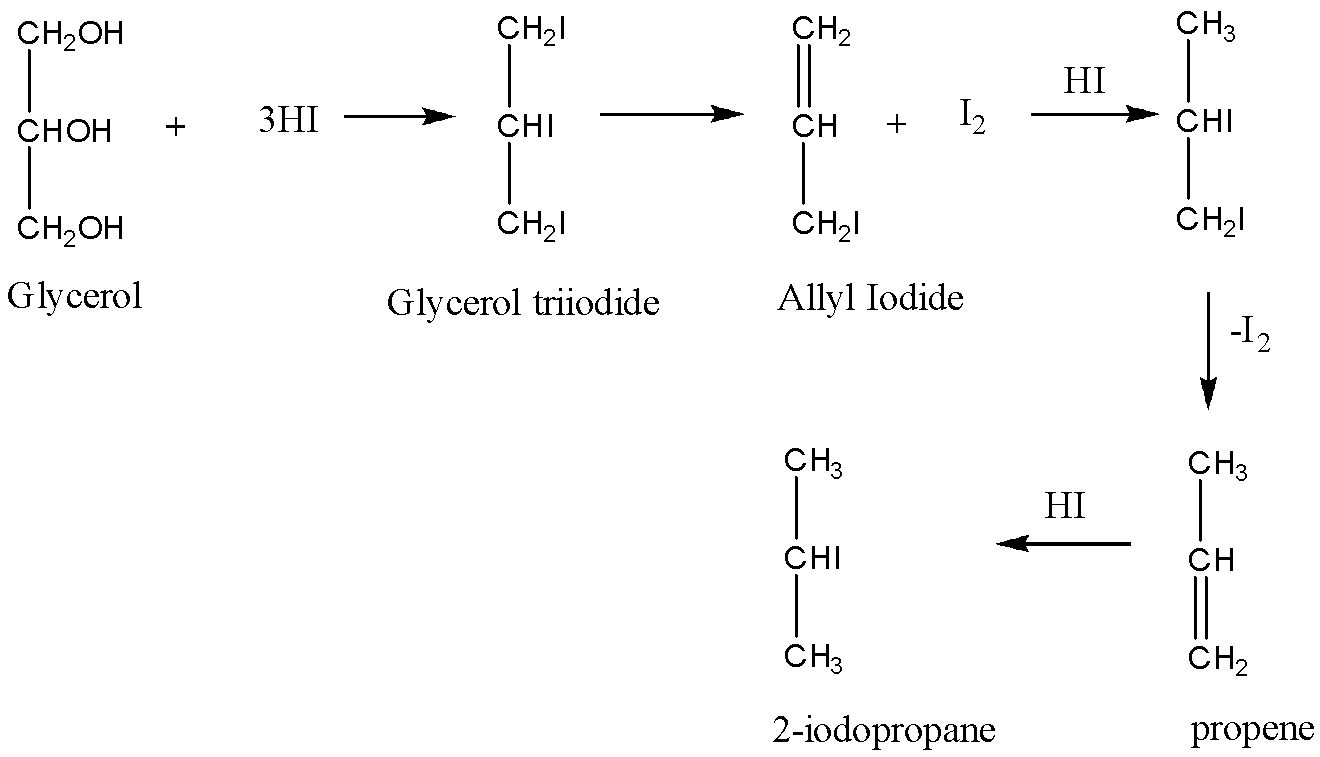
So, the correct answer is 2−iodopropane, Option A.
Note:
In such type of questions, one must answer the final product which is formed, well in this question all of the given options are formed in the reaction either as an intermediate or as a final product, in the first step the glycerol triiodide is formed followed by the formation of allyl iodide and then followed by propene and finally the 2−iodopropane.
