Question
Question: When formaldehyde is treated with ethylamine, it gives: A. ethyl alcohol B. propionaldehyde C....
When formaldehyde is treated with ethylamine, it gives:
A. ethyl alcohol
B. propionaldehyde
C. dimethylamine
D. ethyl methyl imine
Solution
Formaldehyde is the simplest aldehyde, which is the organic compound with (CHO) functional group. Ethyl amines are the compounds having the functional group amine(−NH2). The reaction between aldehyde and amine is a type of reversible reaction that results in the formation of imines having the CH=NH group.
Complete answer:
The reaction between formaldehyde, the simplest aldehyde and the amine functional group that is a derivative of ammonia is carried by a reversible process. Formaldehyde and ethylamine react together in the presence of acid to form a compound called substituted imine that has the CH=NH group. This imine is also known as a Schiff base.
The reaction between formaldehyde and ethyl amine is as follows:
HCHO+C2H5NH2H2C=NC2H5+H2O
The compound formed is known as ethyl methyl imine. The mechanism of the reaction involves a reversible reaction, but due to the formation of water, it will shift in the forward direction. The mechanism is:
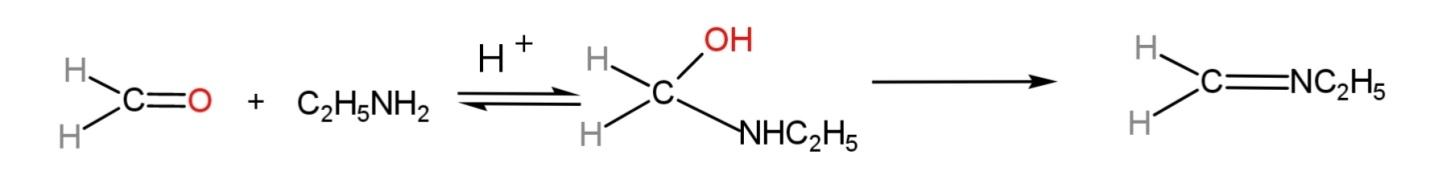
Hence, formaldehyde treated with ethyl amine gives ethyl methyl imine.
So option D is correct.
Note:
The process is reversible because ammonia and its derivatives can be decomposed in acid or alkali giving back pure aldehyde. The imine formed here is a Schiff base that is used in various organic syntheses and also as intermediate or catalysts. Schiff bases also act as important ligands in coordination compounds. Ketones also react in the same way as aldehyde to form keto imines.
