Question
Question: When ethanamide is treated with \(EtMgBr\), followed by hydrolysis, the product is: (A)  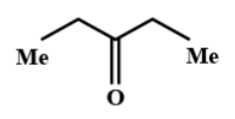
(B) 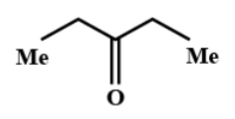
(C) 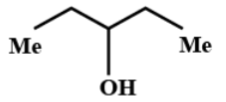
(D) 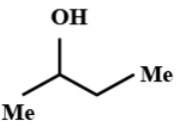
Solution
A Grignard reagent allows us to take any hydrocarbyl halide, metallate it, and when it tends to react with a carbonyl compound it will form carbon-carbon bonds straightforwardly and directly. When it reacts with an amide, on hydrolysis it will try to form an ether.
Complete step by step solution:
As per the given question, ethanamide (with chemical formula CH3CONH2) reacts with EtMgBr that is ethyl magnesium bromide, which is a Grignard reagent and it also undergoes hydrolysis. So, to find out the product let us see what is the Grignard reaction and what is the mechanism of the reaction.
So, the Grignard reaction, discovered by Francois Auguste Victor Grignard refers to an organometallic chemical reaction where groups like alkyl, allyl, vinyl, or aryl-magnesium halides when are added to a carbonyl group tends to form carbon-carbon bond.
Grignard reagent is highly nucleophilic in nature. So, this reagent will generally attack the electrophilic carbon in the polar bond of the carbonyl group and will further tend to form a carbon-carbon bond structure.
Thus, here considering the Methyl group in ethanamide as the R group and the methyl group in EtMgBr as the R′group, when it is hydrolysed it tends to form a ketone and eliminates the −NH2 group. So, the reaction will be like as shown in the below figure:
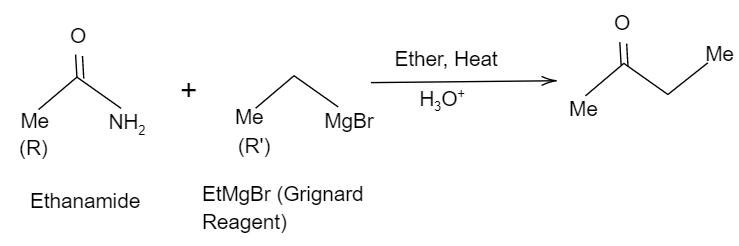
Hence, the correct option is B.
Note: The reaction of alkyl or aryl halides gives the Grignard reagent as product and is generally used for the synthesis of aldehydes and ketones (especially). The Grignard reaction is also used for the synthesis of carbon-carbon bonds.
