Question
Question: When cyclohexanone reacts with selenium dioxide, what product will be formed? 
A.Cyclohexane 1,2− dione
B.1,6− hexanedione
C.Cyclohexane 1,4− dione
D.2− hydroxy cyclohexan 1− one
Solution
Functional group: In the hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound. And if CO is attached then the functional group will be ketone.
Complete step by step solution:
First of all we will talk about the alkanes, alkenes and alkynes.
Alkanes: The compounds which are formed by carbon and hydrogen and have only a single bond between the carbon-carbon atoms, are known as alkanes. For example: The first member of the alkane family is ethane (H3C−CH3). The general formula of the alkane group is CnH(2n+2).
Alkenes: The compounds which are formed by carbon and hydrogen and have at least one double bond along with a single bond between the carbon-carbon atoms, are known as alkenes. For example: The first member of the alkene family is ethene (H2C=CH2). The general formula of the alkene group is CnH2n.
Alkynes: The compounds which are formed by carbon and hydrogen and have at least one triple bond along with a single bond between the carbon-carbon atoms, are known as alkynes. For example: The first member of the alkyne family is ethyne (HC≡CH). The general formula of the alkyne group is CnH(2n−2).
| Number of carbon atom in alkane | Name of the parent chain |
|---|---|
| One | Methane |
| Two | Ethane |
| Three | Propane |
| Four | Butane |
| Five | Pentane |
| Six | Hexane |
| Seven | Heptane |
Functional group: In hydrogen the atoms or groups which are other than carbon and hydrogen, are known as function groups. For example: chloride if chlorine is present in the compound.
When cyclohexanone reacts with selenium dioxide, then the product is as Cyclohexane 1,2− dione. Because the cyclohexane ring will be the same and one more ketone group will be attached to the parent chain so the IUPAC name of the product will be Cyclohexane 1,2− dione. The reaction is as follows:
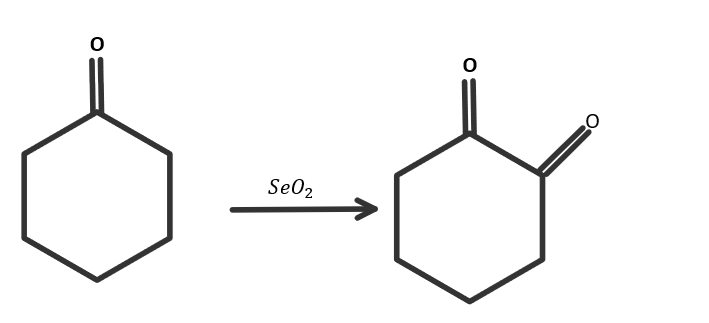
So the correct answer is option C.
Note: Suffix to some functional group are as: for carboxylic acid suffix used is –oic acid, for alcohols suffix used is alkyl alcohol. For example: if an alcohol group is present in methane then the IUPAC name of the compound will be methyl alcohol.
