Question
Question: When benzene in presence of anhydrous aluminium chloride reacts with ethyl chloride the compound for...
When benzene in presence of anhydrous aluminium chloride reacts with ethyl chloride the compound formed is:
A.
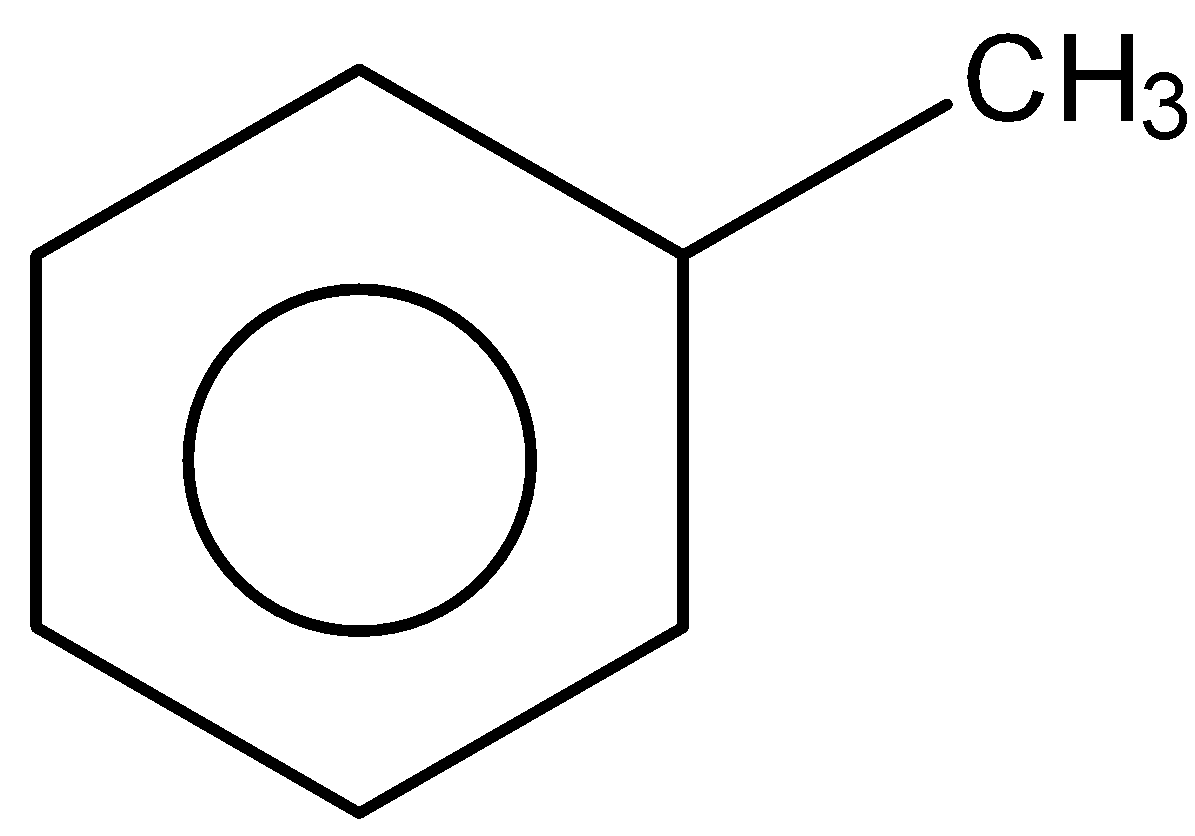
B.
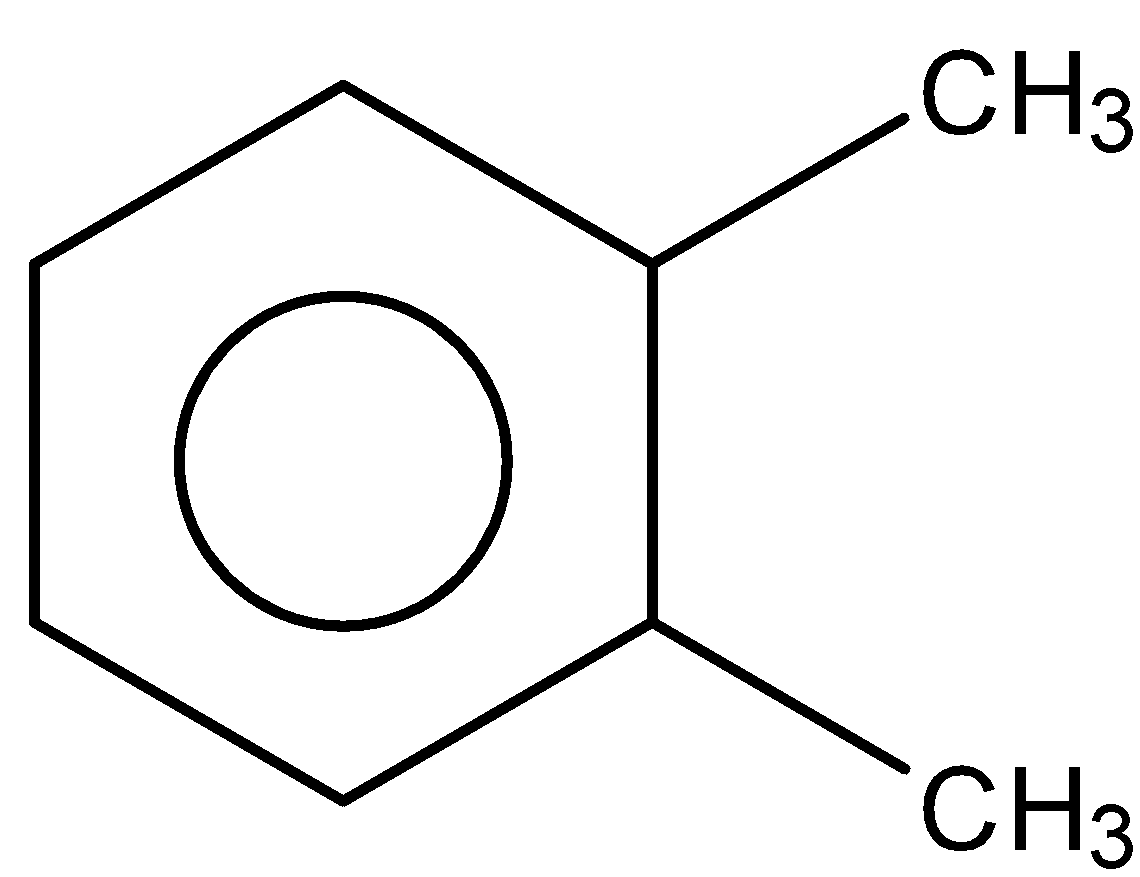
C.
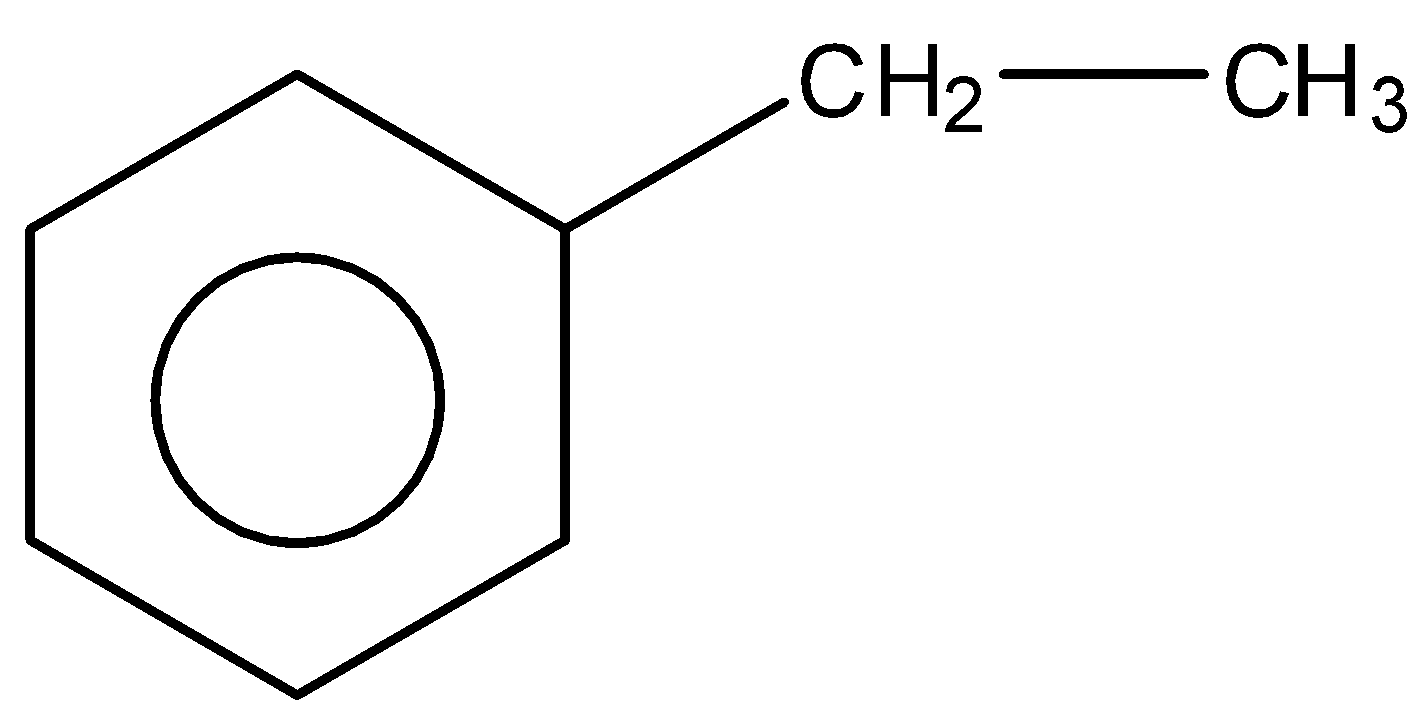
D.
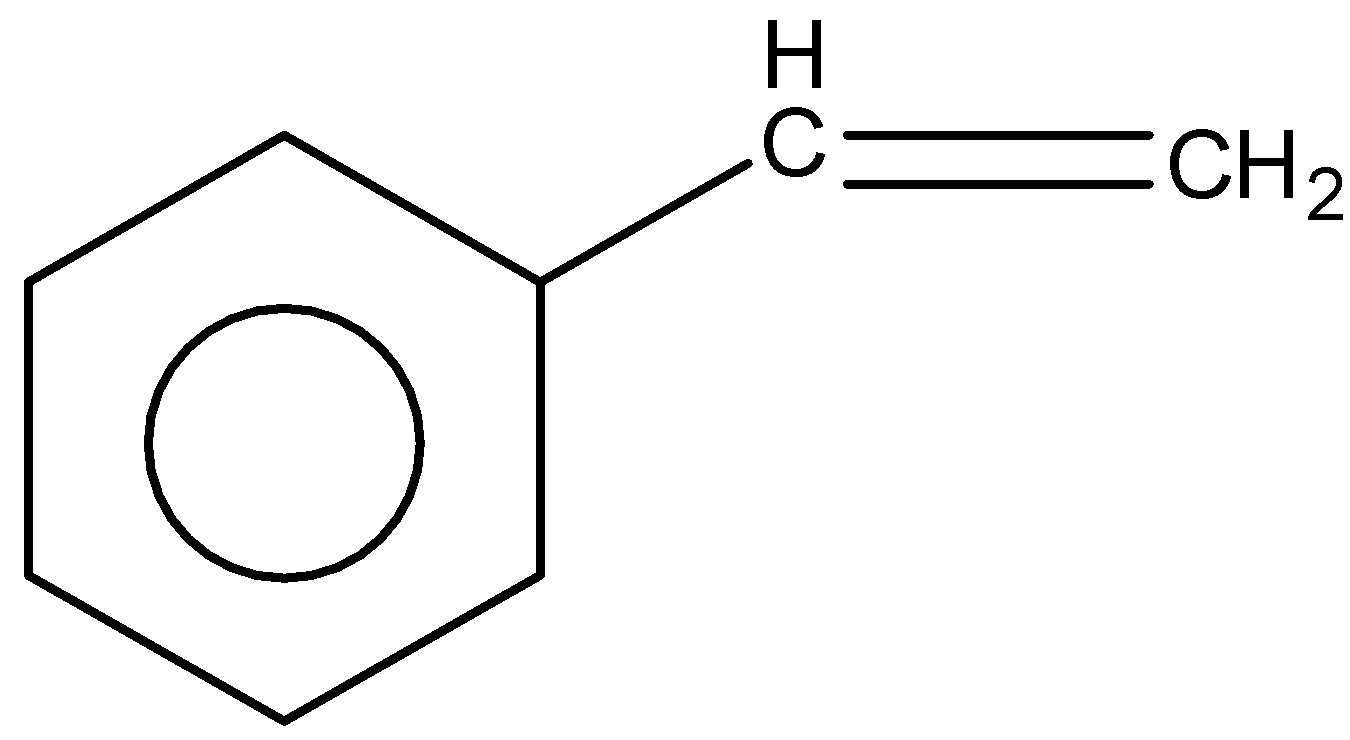
Solution
We know that haloarenes usually undergo electrophilic reactions of benzene rings. Some examples of electrophilic reactions of benzene are nitration, halogenations, Friedel Crafts reaction and halogenations reaction.Friedel Crafts reaction in detail. It is the reaction in which benzene undergoes reaction with alkyl halide (typically bromide, chloride or iodide) in presence of a Lewis acid namely aluminium chloride (AlCl3), ferric chloride (FeCl3)etc.
Complete step by step answer:
Let se Friedel Crafts reaction
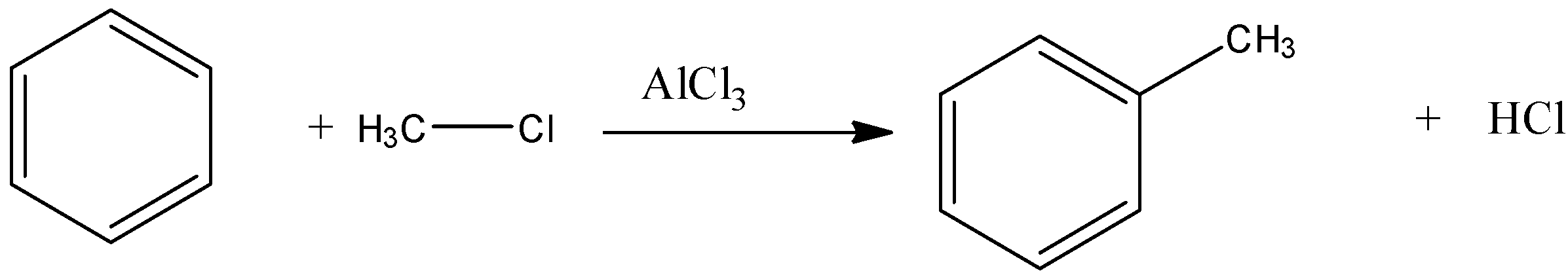
Now, come to the question. Here, benzene reacts with ethyl chloride in presence of anhydrous aluminium chloride. That means, the reaction is a Friedel Craft reaction.
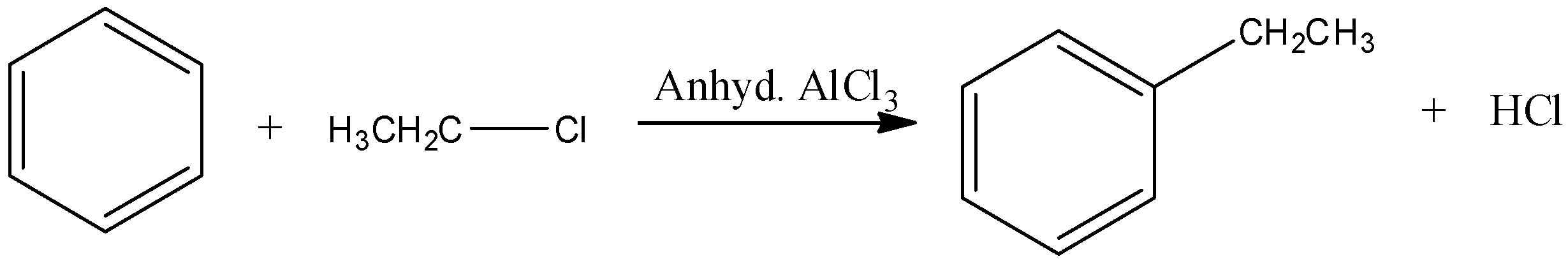
Let’s understand the electrophilic substitution of benzene rings. Halogen atom is o,p- directing besides being slightly deactivating. Therefore, substitution occurs at para and ortho position with respect to the halogen.
The o, p influence of halogen atoms is understood by the resonance structures of a halobenzene (chlorobenzene). The resonance structures of chlorobenzene are,
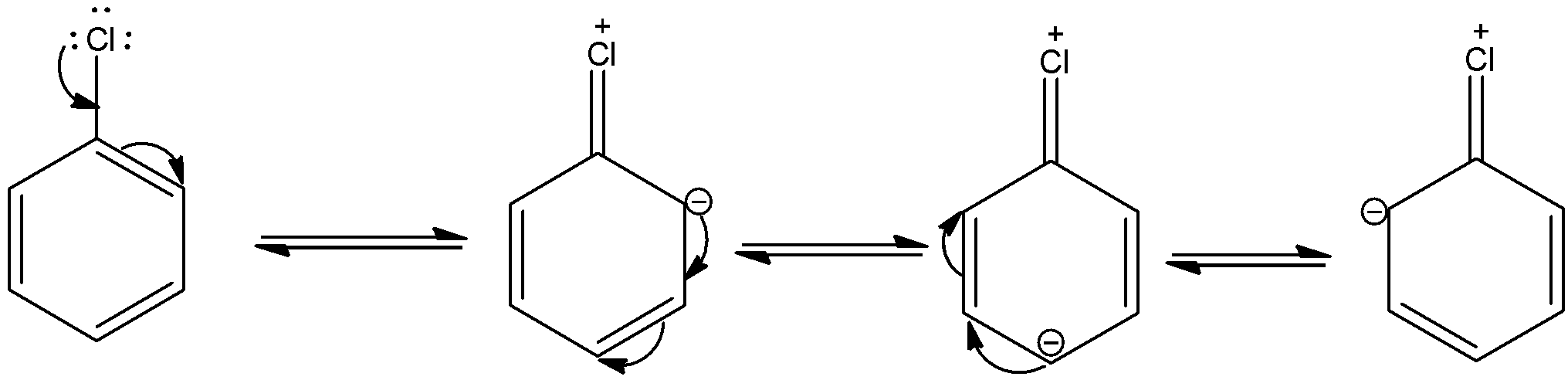
Due to resonance, electron density increases at ortho and para positions than at meta position. Also, the halogen atom shows –I effect due to which it has a tendency to withdraw electrons from the benzene ring. Because of this, the ring gets deactivated comparing the benzene ring. This is the reason due to which electrophilic substitution in haloarenes occurs slowly and needs drastic conditions compared to benzene.
So, the correct answer is Option c.
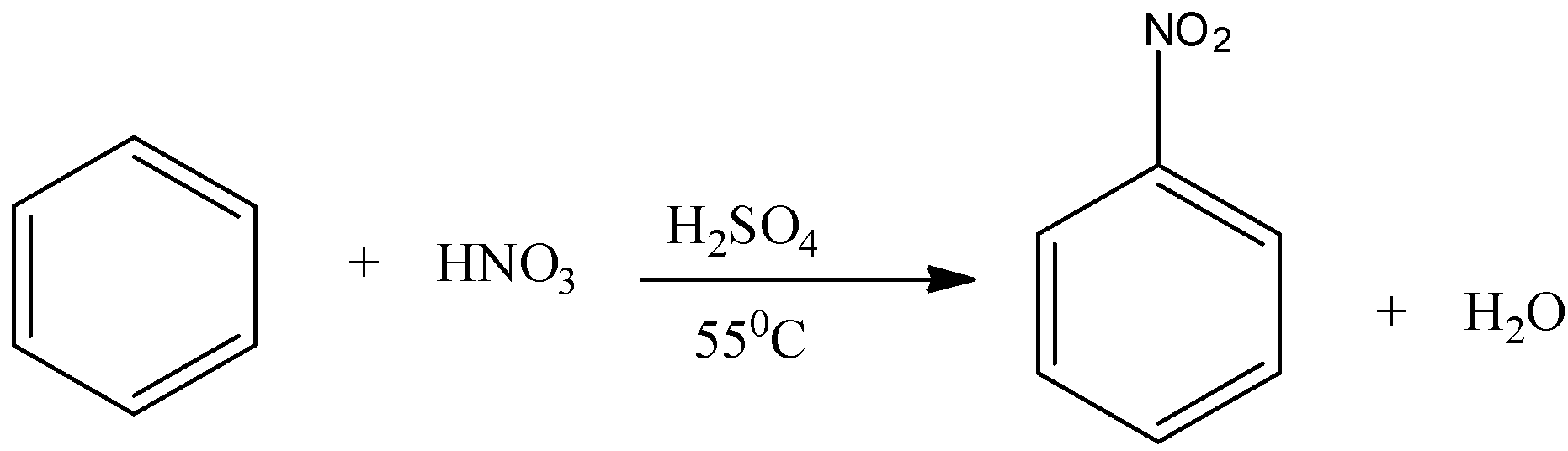
Note: Nitration is the reaction in which a nitrate group is introduced to the benzene ring. This can be done by reacting benzene with concentrated nitric acid at 55 degree Celsius in presence of the catalyst, that is, concentrated sulphuric acid.