Question
Question: When baking soda reacts with vinegar, is it formed. A.sodium carbonate B.sodium hydrogen carbo...
When baking soda reacts with vinegar, is it formed.
A.sodium carbonate
B.sodium hydrogen carbonate
C.sodium acetate
D.none of above
Solution
Baking powder is a dry chemical leavening agent, made up of an acid, base, and buffering material. This buffering material prevents the reaction of acid and base before its intended use. For instance, corn-starch is a common buffering material. Baking powder is a dry chemical leavening agent, made up of an acid, base, and a buffering material. This buffering material prevents the reaction of acid and base before its intended use. For instance, corn-starch is a common buffering material.
Complete step by step answer:
Generally, baking soda can be prepared from soda ash or sodium carbonate. It is also known as sodium bicarbonate (NaHCO3) Baking soda is used for baking, cleansing, as a medicinal ingredient, as a fire retardant, etc. baking soda can also absorb moisture from the air that is why it is used in the refrigerator. On thermal decomposition baking, soda is converted into soda ash by releasing carbon dioxide.
NaHCO3→Na2CO3 + H2O + CO2
Vinegar is a naturally occurring compound that is in the liquid state, so there is no defined concentration for it. Although we have a range of about 5% to 20% concentration of acetic acid in water. The chemical formula for vinegar thus obtained is CH3COOH . So, vinegar is a diluted solution of acetic acid in water. Because of this, vinegar is considered to be a weak acid. The molecular structure of vinegar can be given as:
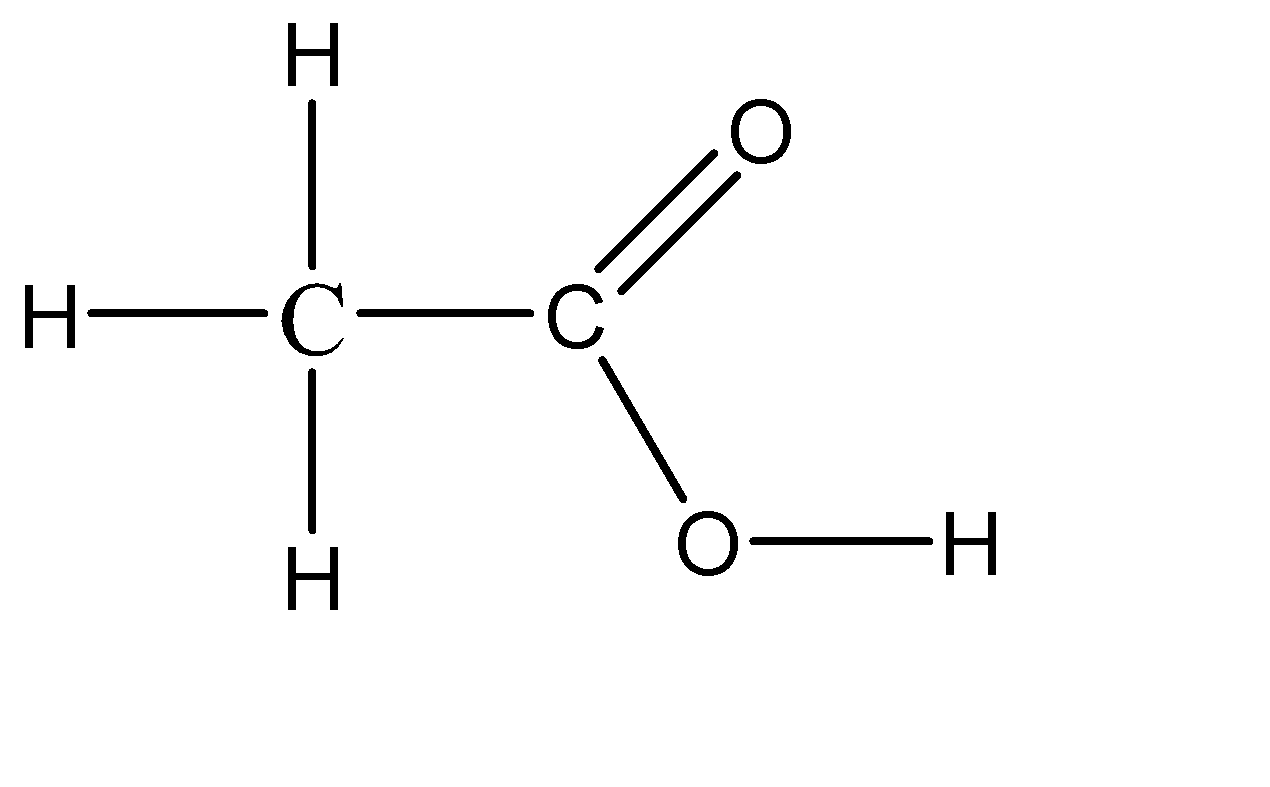
Hence, the acid used in the making of vinegar is acetic acid.
baking soda is a base and vinegar is acid. Therefore, when baking soda reacts with vinegar acid-based reaction will happen. Any acid-base reaction is exothermic in nature.
NaHCO3+CH3COOH→CH3COONa+CO2+H2O
So, the correct answer is C.
Note: Sodium bicarbonate in presence of acid when combined with water, breaks down to carbonate and releases carbon dioxide, making the cake rise by creating air bubbles in it. Baking powder increases the volume of the cake and lightens the texture of baked sponge through an acid-base reaction, thus leavening the batter.
That is why the absence of baking powder results in hard and small-sized cakes. Some good substitutes for baking powder in case not accessible are- buttermilk, plain yogurt, baking soda with vinegar, and lemon juice. They work the same as baking powder.
