Question
Question: When acetyl chloride is heated with Na salt of carboxylic acid, the product is an : A. Ester B....
When acetyl chloride is heated with Na salt of carboxylic acid, the product is an :
A. Ester
B. Anhydride
C. Alkene
D. Aldehyde
Solution
Nucleophilic substitution reactions are those reactions in which substitution is brought about by a nucleophile. These reactions are denoted by SN (S stands for substitution and N for nucleophile).
Complete step by step solution:
When acetyl chloride is heated with Na salt of carboxylic acid, the product is an acetic anhydride (which is an anhydride).
The following reaction takes place:
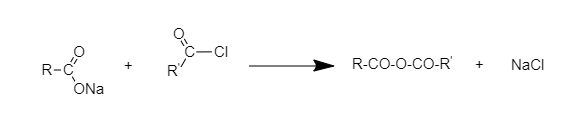
Hence, the correct option is B.
Electrophiles and Nucleophiles are atoms, bonds, or (small or small parts of) compounds that are involved in chemical reactions.
Electrophile may be defined as a species which are electron lovers. Such species are electron deficient in nature. It may be neutral or +ve charged.
The nucleophile may be defined as a species, which is electron-rich in nature. Such species are neutral or –ve in charge.
Stabilization of the negative charge on the acyl ion. The higher the stabilization through resonance, the lower will be the electrophilic nature and hence its reactivity.
The lower the partial positive charge on the carbonyl atom, the lower will be its electrophilicity and hence reactivity.
Nucleophilic substitution reaction is the reaction in which the nucleophile substitutes the electrophile.
Note: The possibility to make a mistake is that you may choose option C. But when acetyl chloride is heated, an anhydride is formed not an alkene.
