Question
Question: When acetamide is treated with \(B{r_2}\) and caustic soda, the product formed is: (A) N-bromamide...
When acetamide is treated with Br2 and caustic soda, the product formed is:
(A) N-bromamide
(B) bromoacetic acid
(C) methanamine
(D) ethanamine
Solution
This reaction of acetamide / amide with bromine in presence of caustic soda is known as Hoffmann bromamide reaction. In this reaction, the product formed has less carbon than the starting reactant .
Complete step by step answer:
Now let us first understand about acetamide. Acetamide has an IUPAC name Ethan amide. It is an compound with chemical formula C2H5NO Its structure is as follow:
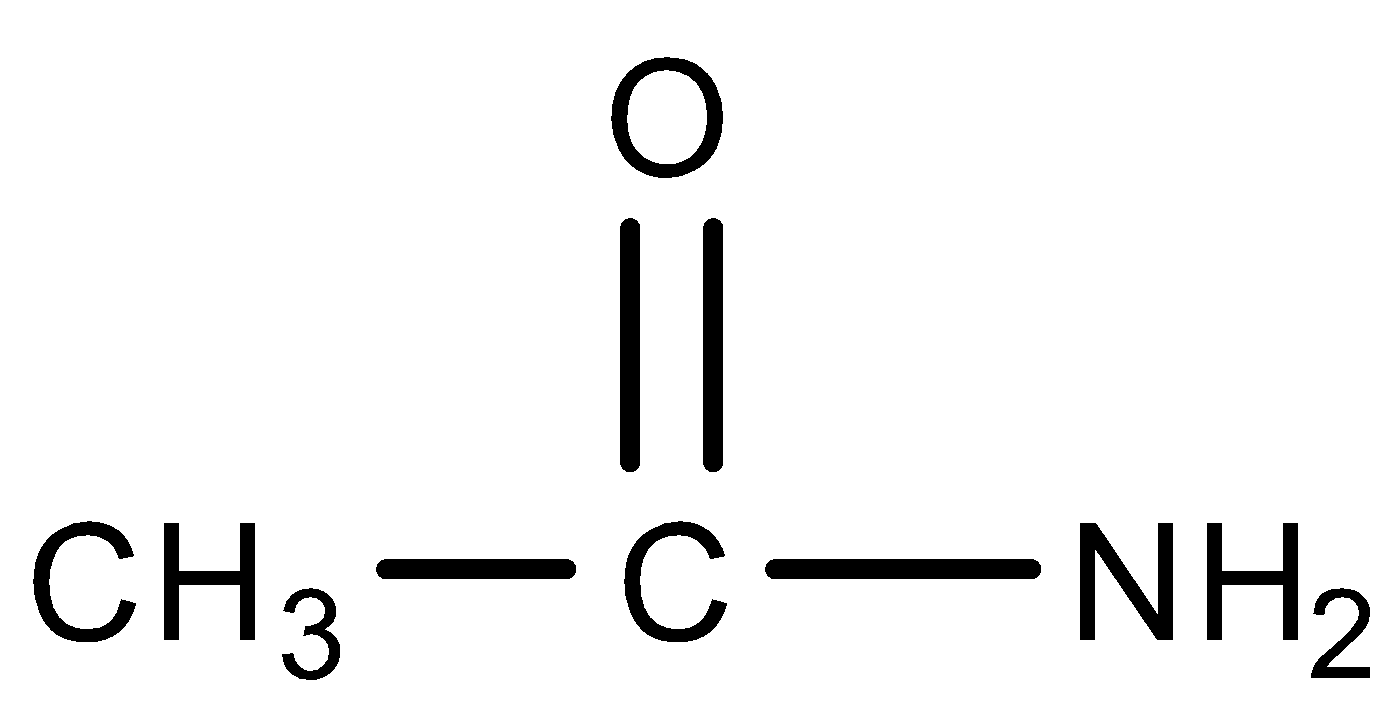
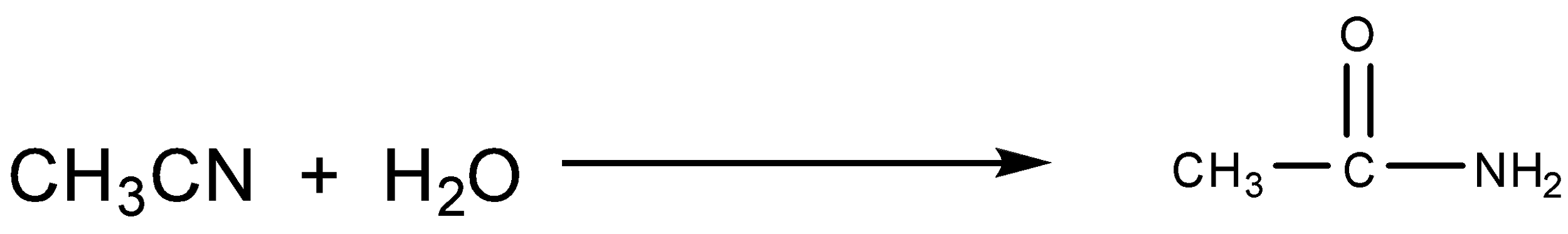
Acetamide is the simplest amides derived from acetic acid. Atomic is an industrial solvent and is used as a plasticizer, its dielectric constant is higher than most organic solvent, allowing it to dissolve inorganic compounds. Acetamide is also used in pesticides, pharmaceuticals and antioxidants for plastics. On an industrial scale, acetamide is produced by the hydration of acetonitrile.
Moreover, we know that caustic soda is commonly known as sodium hydroxide (NaOH) . It is a white solid ionic inorganic compound.
When acetamide is treated with Br2 and caustic, it fields methanamine, as the main product. This reaction of treating amide with bromine in the presence of alcoholic KOH alcoholic NaOH to field an amine, with one carbon atom less than the starting amides is known as Hoffmann bromamide reaction. The reaction of acetamide with Br2 and caustic soda is as follow
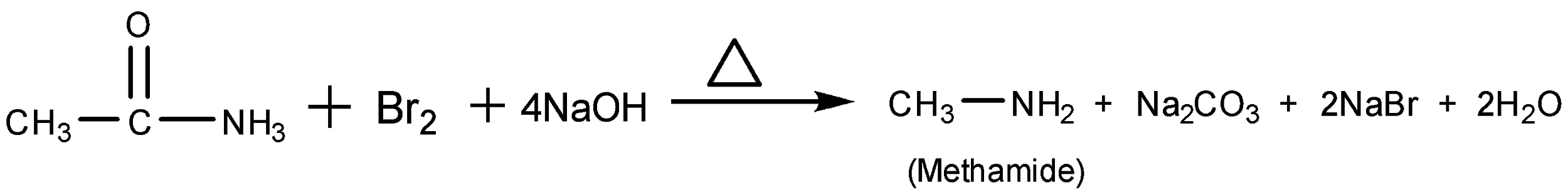
So, the correct answer is Option C.
Note: In Hoffmann bromamide reaction, only 1∘ amines are formed. 2∘ and 3∘ amines cannot be formed by this reaction . Moreover, always remember that the product in this reaction product contains carbon atoms less than the starting amide.
