Question
Question: When acetaldehyde is reacted with \[LiAl{{H}_{4}}\], What is the product formed? A.\[C{{H}_{3}}COO...
When acetaldehyde is reacted with LiAlH4, What is the product formed?
A.CH3COOH
B.CH3CH2OH
C.CH3OH
D.HCOOH
Solution
The molecular formula of acetaldehyde is CH3CHO. Lithium aluminium hydride (LiAlH4) is a reducing agent which facilitates the reduction of the reacting substrate. Reduction describes the addition of hydrogen.
Complete answer:
The molecular formula of acetaldehyde is CH3CHO. Lithium aluminium hydride (LiAlH4) is a reducing agent which facilitates the reduction of the reacting substrate. Reduction describes the addition of hydrogen. Thus, the reduction of aldehyde in presence of LiAlH4 lead to the formation of alcohol. This is illustrated in the following reaction:
CH3CHO+LiAlH4→CH3CH2OH
The general reaction mechanism for the above reduction is given as:
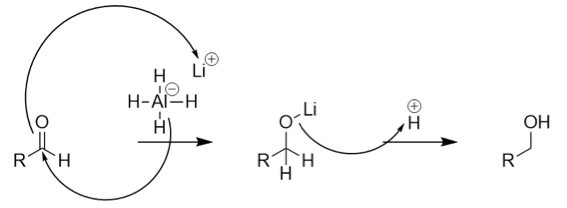
From the above mechanism, we conclude that the reduction of aldehyde takes place via hydride transfer. Upon reduction, the number of carbon atoms in the moiety remains the same. Therefore, option (B) is correct. In contrast, option (A) contains CH3COOH which is the oxidised form of acetaldehyde thus this option is incorrect. Option (C) contains CH3OH which contains less number of the carbon atoms than acetaldehyde. Thus, option (A) is incorrect. Option (D) contains HCOOH which is the oxidised form of formaldehyde instead of acetaldehyde. Thus, option (D) is incorrect.
From the above discussion, we conclude that option (B) is correct.
Note:
It is important to note that acetaldehyde undergoes reduction when reacted with LiAlH4. This reduction takes place via hydride transfer. The final product will be CH3CH2OH and hence option (B) is correct.
