Question
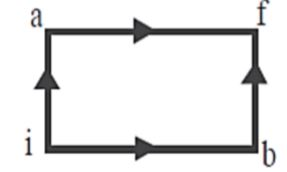
Question: When a system is taken from state \[{\text{i}}\] to state \[{\text{f}}\] along the path \[iaf\], it ...
When a system is taken from state i to state f along the path iaf, it is found that Q=50 cal and W=20 cal . Along the path ibf, Q=36 cal, W along the path ibf is:
A. 6 cal
B. 16 cal
C. 66 cal
D. 14 cal
Solution
In order to answer this question, we need to understand the first law of thermodynamics. First law of thermodynamics states that we know that both the total work done W and the total heat transfer Q to or from the system depend on the thermodynamics path. However, the difference Q−W is small for all paths between the given initial and final equilibrium states, and it is equal to the change in internal energy ΔU of the system. Using this law and making some modification to the law we can get the answer.
Formula Used:
ΔU=Q−W
Where, ΔU is the change in the internal energy of the system, W is the work done by the system on the surrounding, Qis the heat supplied to the system on the surrounding.
Complete step by step answer:
First law states that whenever heat is being added in a system from the external source. Some of the energy stays with the system, and the rest of it gets consumed from work. The energy left in the system increases the internal energy. Internal energy is the total of your kinetic energy and potential energy. According to the first law of thermodynamics for the path iaf,
Qiaf=ΔUiaf+Wiaf
Rearranging the terms,
ΔUiaf=Qiaf−Wiaf
Here we know that,
Qiaf=50
Wiaf=20
Substituting the values we get,
ΔUiaf=Qiaf−Wiaf
⇒ΔUiaf=50−30
⇒ΔUiaf=30
For the path ibf,
Qibf=ΔUibf+Wibf
Since, ΔUibf=ΔUiaf changes in internal energy are path independent.We know,
Wibf=Qibf−ΔUibf
Substituting the values we get,
Qibf=36 cal
⇒ΔUibf=30 cal
⇒Wibf=36−30
∴Wibf=6 cal.
Hence, the correct option is (A).
Note: It should be remembered that there are four main laws of thermodynamics, but in most cases, we only need the first three. In addition to this, the potential of molecular networks and every other minute detail of its design can be studied using thermodynamics. When heat is supplied to the system, then ΔQ is positive and when heat is withdrawn from the system, ΔQ is negative.
