Question
Question: When a spring is wound, a certain amount of PE is stored in it. If this wound spring is dissolved in...
When a spring is wound, a certain amount of PE is stored in it. If this wound spring is dissolved in acid the stored energy.
A. Is completely lost
B. Appears in the form of electromagnetic waves.
C. Appears in the form of heat raising the temperature of the acid
D. Appears in the form of KE by splashing acid drops.
Solution
We know that spring possesses elasticity property. When spring gets compressed, mechanical energy is stored. This stored mechanical energy leads to an increase in the kinetic energy of molecules of acid present near the string. Use the relation of temperature with kinetic energy and calculate the answer.
Complete answer:
We have been provided with a spring which is wound or compressed and when it gets compressed a certain amount of potential energy is stored in the compressed string. Now this compressed string is dissolved in acid. We have to find out the effect on stored energy in compressed string. Consider a tank fuel of acid.
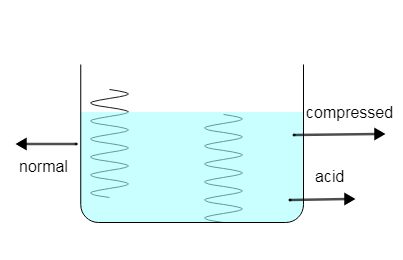
Put two strings inside the tank one spring is compressed and the other one is normal spring with no compression considered, compressed spring compressed spring has mechanical energy therefore it will dissolve faster i.e. rate of dissolving is more.
As we know when the spring compresses it gains mechanical energy due to these mechanical energy acid molecules present near the spring achieves the kinetic energy and hence the kinetic energy of molecules will increase when kinetic energy increases temperature also increases near the string.
Now consider uncompressed spring since normal or uncompressed spring is not stretched by external force, therefore it will not gain mechanical energy therefore it will dissolve by normal rate. No mechanical energy to kinetic energy achieved by molecules of acid.
Hence, when wound springs dissolve in acid the stored energy appears in the form of heat raising the temperature of the acid.
So, the correct answer is “Option C”.
Note:
Whenever molecules of liquid acquire temperature the molecular spacing between the molecules increases and they do possess kinetic energy because of kinetic energy these molecules collide on each other. When the spring gets compressed, because of elasticity property, it gains elastic potential energy.
