Question
Question: When \( 2OH \) combines with another \( 2OH \) would it form an OH subscript \( 4 \) ?...
When 2OH combines with another 2OH would it form an OH subscript 4 ?
Solution
Hint : When two elements combined together they form a molecule. Also, an element forms bonds with other elements on the basis of their outermost electrons. Like, Beryllium (Be) can form only two stable bonds since it has only two outermost electrons.
Complete Step By Step Answer:
Here, we are asked that if 2OH combines with 2OH then whether it will form (OH)4 or not. So, for that let’s write it down in the form of a reaction:
2OH+2OH→(OH)4
And now let’s see the elements individually. Here, Oxygen and Hydrogen are present and we know that Oxygen is an element with atomic number (Z)=8 and atomic mass (A)=16 . So, it’s electronic configuration can be written as [He]2s22p4 .
Now, we know that in p-orbital only 6 electrons can be filled, so as we can see in the electronic configuration of oxygen there are only 4 electrons present which means that Oxygen is capable of forming 2 bonds with other elements.
We also know that Hydrogen is an element with atomic number (Z)=1 and atomic mass (A)=1 . So, it’s electronic configuration can be written as 1s1 .
Now, we know that in s-orbital only 2 electrons can be filled and as we can see from the electronic configuration of Hydrogen there is only 1 electron present which means that Hydrogen can form only one bond with other elements.
And if we look at the above equation which we have written and the explanation then (OH)4 cannot be formed since oxygen can only form two bonds at a particular time and Hydrogen can only form one bond at a particular time. So, it is impossible that the formation of (OH)4 will take place.If we write down the structure of (OH)4 , then it will look like this: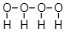
We can get 4OH which means that there is no reaction that took place.
Note :
Here, in this structure (OH)4 cannot be formed because that would not be stable. Therefore, it is impossible to form such types of molecules. Elements form bonds on the basis of their valence electrons, that is, their outermost electrons.
