Question
Question: When 2-ethyl-3-methyl-1-pentene is treated with CH3OH in H2SO4, how many different methoxy ethers wo...
When 2-ethyl-3-methyl-1-pentene is treated with CH3OH in H2SO4, how many different methoxy ethers would be formed in significant amount?
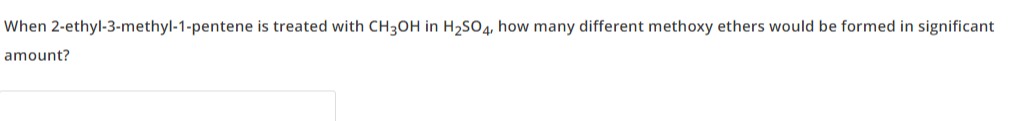
Answer
3
Explanation
Solution
The reaction proceeds via carbocation intermediates. The initial secondary carbocation undergoes 1,2-hydride shift and 1,2-methyl shift to form two different, more stable tertiary carbocations. All three carbocations (the initial secondary and the two rearranged tertiary) react with methanol to form distinct methoxy ethers. Therefore, three different structural isomers of methoxy ethers are formed.
