Question
Question: When \(0.2M\) acetic acid is neutralized with \(0.1M\) NaOH in \(0.5\) liters of water, the resultan...
When 0.2M acetic acid is neutralized with 0.1M NaOH in 0.5 liters of water, the resultant solution is slightly alkaline. Calculate the pH of the resulting solution given that Ka for CH3COOH=1.8×10−5
Solution
The reaction between acetic acid and sodium hydroxide produces sodium acetate and water. Calculate the amount of sodium acetate and water produced in this reaction and find the concentration of sodium acetate accordingly. Now calculate the value of hydrolysis constant or Kh to find the pH value.
Complete step-by-step solution: The above reaction between acetic acid and sodium hydroxide can be written as follows.
CH3COOH+NaOH⇄CH3COONa+H2O
0.2M of acetic acid would give 0.2M of sodium acetate and 0.5 liters of water. So the concentration of sodium acetate would be 0.1molL−1
Given to us the association constant of acetic acid as Ka=1.8×10−5
Let us write the association reaction for acetic acid CH3COO−+H2O⇄CH3COOH+OH−
For the above equilibrium reaction, we can write the concentrations as:
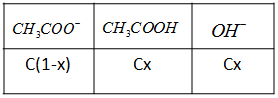
From this, we can write the value of hydrolysis constant as Kh=C(1−x)Cx×Cx=1−xCx2
Since the value of x is extremely small, we can approximate the value of 1−x to be 1 and hence the equation becomes Kh=Cx2
Now, we know that Kh=KaKw where Kw=10−14 and the Ka value is already given. By substituting these values, we get Kh=1.8×10−510−14=0.55×10−9
From this, we can write Cx2=0.55×10−9
We have already calculated the concentration as 0.1molL−1 and by substituting this, we get x2=5.5×10−9
This gives x=7.42×10−5
From the above table, the concentration of OH− is Cx=0.1×7.42×10−5=7.42×10−6
We know that the product of concentration of H+ and OH− of a solution gives the value of dissociation constant of water.
[H+][OH−]=Kw
From this, we can calculate the concentration of H+ as [H+]=[OH−]Kw=7.42×10−610−14=1.347×10−9M
Now we can calculate the pH value as −log[H+]=8.87
Hence the pH value is 8.87
Note: It is to be noted that we can also find the value of pOH first and derive the value of pH. This is because of the relation pH+pOH=14 and hence the value of pH can be calculated. Therefore the pOH of this solution would be 14−pH which is 14−8.87=5.13 .
