Question
Question: What would be the product for the following reaction? 
A. Ph−Cl
B. Ph−N2
C. 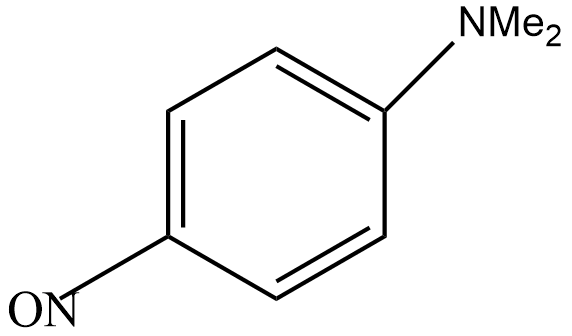
D. 
Solution
Since it is tertiary aromatic amines that means there is no N-H bond s nitrosylation on nitrogen and salt formation is not possible. This reaction is used for the distinction of primary, secondary and tertiary amines.
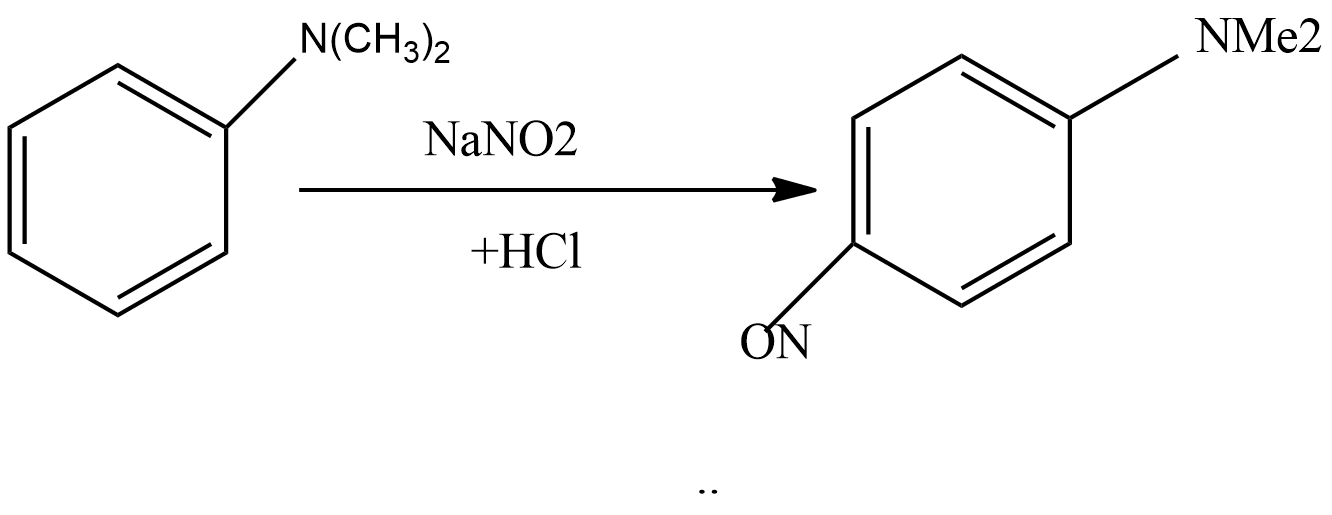
Complete step by step answer: In this reaction, there is electrophilic substitution at the para position. Below are the steps involved in the reaction:
Step1: Sodium nitrite reacts with hydrochloric acid to form sodium chloride and nitrous acid. The reaction for this step is written as:
NaNO2+HCl→NaCl+HNO2
Step2: This is the protonation step, below is the reaction that shows protonation
H..O..N=O+H+→H2..O+N=O
Step 3: This step involves the formation of the nitrosonium ion. Since the oxygen atom is one of the most electronegative atoms, so + charge on it makes it unstable so it withdraws the electrons from the nitrogen atom and forms water and nitrosonium ion N+=O.
Step 4: In this step, nitrosonium ion attacks on N, N-dimethylaniline to give N, N-dimethyl-p-nitrosoaniline as the main product.
So, the correct answer is “Option C”.
Additional Information: Electrophilic substitution reactions are that chemical reaction in which an electrophile displaces a functional group in a compound. There are mainly two types of electrophilic reaction:
- Electrophilic aromatic substitution reaction
- Electrophilic aliphatic substitution reaction.
- There are some important reactions of Electrophilic aromatic substitution type that take place are aromatic nitration, aromatic halogenation, aromatic sulfonation, and friedel craft reactions.
Note: Here, the dimethylaniline group activates the benzene ring at ortho and para position. But the steric hindrance of the bulkier group does not attack the ortho position. As a result, the compound formed is known as P-nitrosodimethylaniline. It is a dark green crystalline solid and is insoluble in water.
