Question
Question: What would be the electron dot structure of carbon dioxide which has formula \(C{O_2}\)?...
What would be the electron dot structure of carbon dioxide which has formula CO2?
Solution
Electron dot structure of any molecule can be obtained by the Lewis-Kossel octet rule. In which valence shell electrons are represented around the symbol of an atom.
Complete step-by-step answer: Lewis-Kossel octet rule-
According to Lewis-Kossel octet rule, in the outermost shell of an element there should be eight electrons whenever it participates in the molecule formation. For which they draw a structure of molecules in which valence shell electrons are represented around the symbol of atom and sharing between atoms takes place such that the outermost shell of the atom is completely filled or show electronic configuration equivalent to their nearest noble gas. Lewis-Kossel octet rule states that in a compound constituent atom share, lose or gain electrons to form a stable electronic configuration in which the outermost shell contains 2 or 8 electrons.
Electron dot structure of CO2 can be shown as-
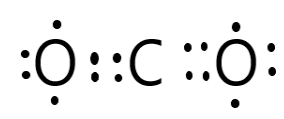
Note: Exception of Lewis-Kossel octet rule-
1.Incomplete octet molecule-
Central atom has less than eight electrons in its outermost shell like BeCl2and BF3
2.Electron efficient molecule-
Molecules in which the central atom acquired more than eight electron in outermost shell e.g. In PCl5 central atom has 10e−.
3.Pseudo inert gas configuration-
Atoms and ions in which outermost shell acquired 9−18e− which gives it extra stability
e.g. ions of transition metal.
4.Inert pair effect-
Mainly shown by the heavier element of 13,14,15 group. Their oxidation state changed by a factor of 2.
e.g. Tl+3 is unstable but Tl+ is stable while
5.Odd electron molecule-
The species in which central atom has unpaired electron or odd number of electron
e.g. NO,NO2
