Question
Question: What will happen if Methyl cyanide and Methyl isocyanide moisture are analyzed by acidic water?...
What will happen if Methyl cyanide and Methyl isocyanide moisture are analyzed by acidic water?
Solution
Hint : Methyl cyanide is the chemical compound which has the formula of CH3CN and cyanide are written as ( −CN ) whereas methyl isocyanides is the chemical compound which has the same chemical formula as of methyl cyanide but isocyanide is written as ( −NC ). They have the net charge i.e. −1 Methyl cyanide is connected to any group via carbon atom whereas Methyl isocyanides are connected via nitrogen atom.
Complete Step By Step Answer:
Hydrolysis of a reaction occurs when the water molecules break one or more chemical bonds and acidic hydrolysis of a reaction occurs when the hydrolysis reaction takes place in the acidic ( H+ ) medium. The acid hydrolysis of cyanide ( −CN ) gives carboxylic acids and of isocyanides gives primary amines.
The hydrolysis of methyl cyanide in the presence of acid gives acetic acid as follows:-
Chemical reaction-
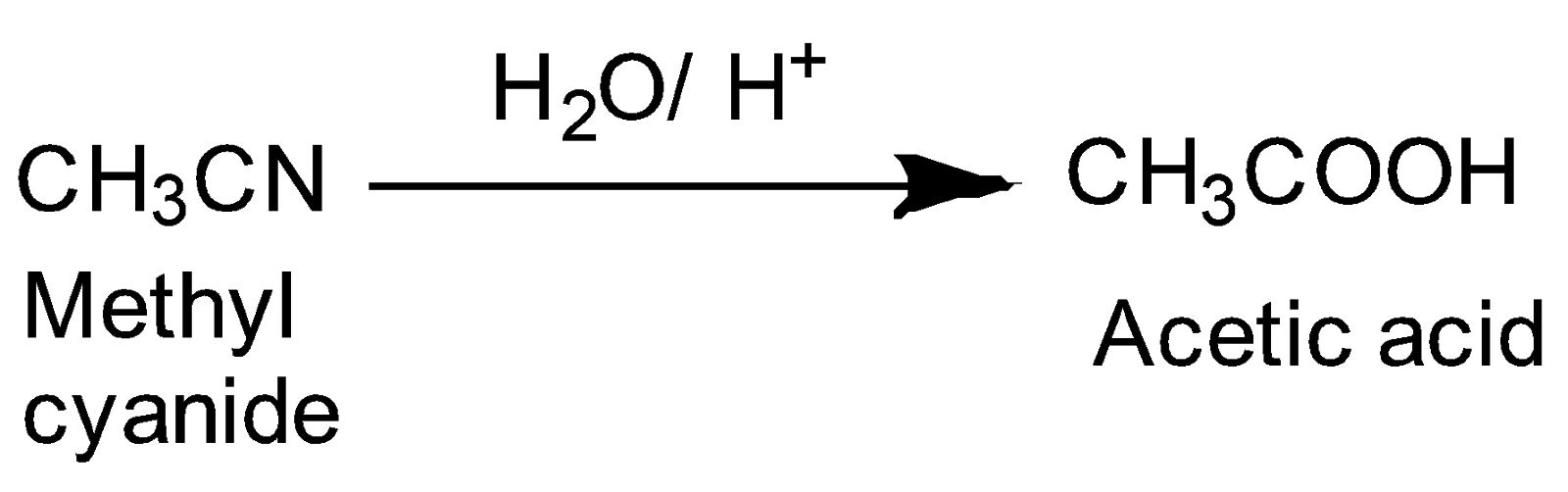
Hence, this reaction results in the formation of carboxylic acids.
Whereas,
The hydrolysis of methyl isocyanide in the presence of acid gives primary amines as follows:-
Chemical reaction-
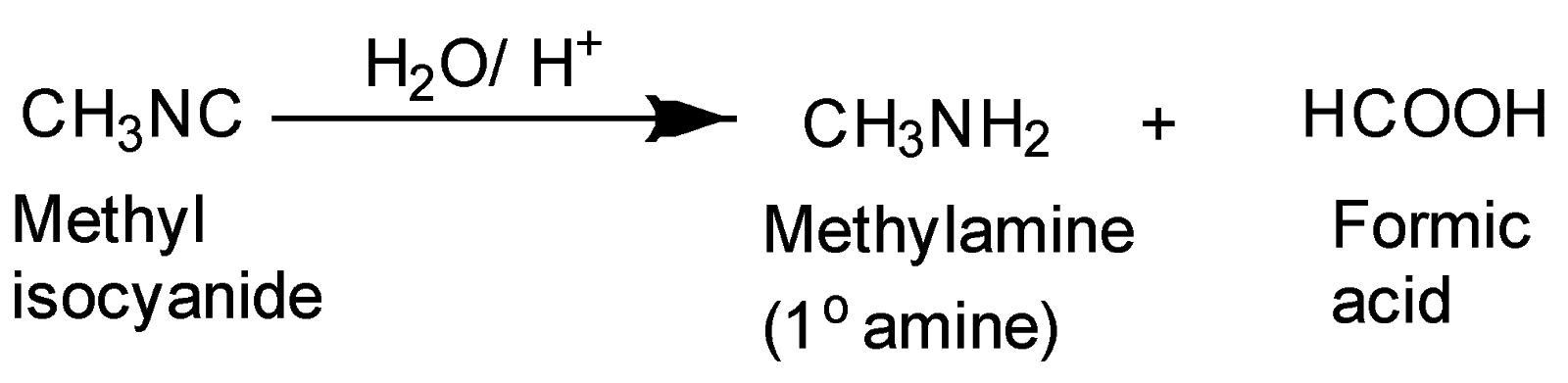
Hence, this reaction results in the formation of primary amine and formic acid.
Additional Information:
Methyl cyanide is more polar and has greater attractive forces due to which the dipole moment of methyl cyanide is higher than methyl isocyanide. Thus, the boiling point methyl cyanide is higher than the methyl isocyanide. Cyanide has a bitter almond odour while isocyanide has extremely unpleasant odour. Isocyanide foul up the air for several days after opening its flask.
Note :
It needs to be remembered that methyl cyanide is also known as acetonitrile and methyl isocyanide is also known as iso cyanomethane. Cyanide ( −CN ) always connects with the group from the carbon atom and isocyanide ( −NC ) always connects with the group from the nitrogen atom but they both have the same chemical formula.
