Question
Question: What will be the product of this reaction? 
Solution
This is a name reaction known as cross Cannizzaro reaction. It occurs between two different aldehydes in the presence of concentrated alkali either NaOH or KOH to give all possible products.
Complete step by step answer:
When formaldehyde reacts with benzaldehyde, the cross Cannizzaro reaction takes place and there is the formation of benzyl alcohol and sodium formate. The chemical equation for the above reaction is written as:
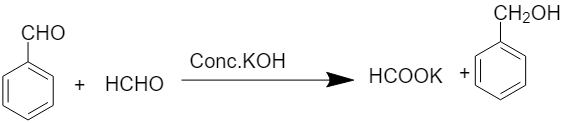
Mechanism of reaction:
In the first step, base attacks the carbonyl carbon of the less bulky aldehyde i.e. formaldehyde to form an addition intermediate as:

In the next step, hydrogen from the addition intermediate shifts to the carbonyl carbon of benzaldehyde.

In the last step, the proton transfer occurs in the following way:

In the case of formaldehyde as one of the reactants, the cross Cannizzaro reaction is of great synthetic utility. For cross Cannizzaro reaction there must be no compulsion of α hydrogen in aldehydes. In this reaction, when aldehydes are treated with an alkali solution, it undergoes a disproportionation reaction, i.e. self-oxidation-reduction reaction. As a result, one of the aldehydes reduces alcohol and formaldehyde oxidized to a carboxylic acid. Formaldehyde oxidizes easily as compared to the other aldehyde because in the case of formaldehyde there is no electron-donating group, the initial nucleophilic addition of hydroxide ion is faster on formaldehyde.
Note:
If we talk about the advantage of cross Cannizzaro reaction, in this reaction both the aldehyde used is entirely converted to products, and wastage of the valuable reactant chemical is avoided. The atom economy of the process is also low.
