Question
Question: What will be the product of reaction? 
A.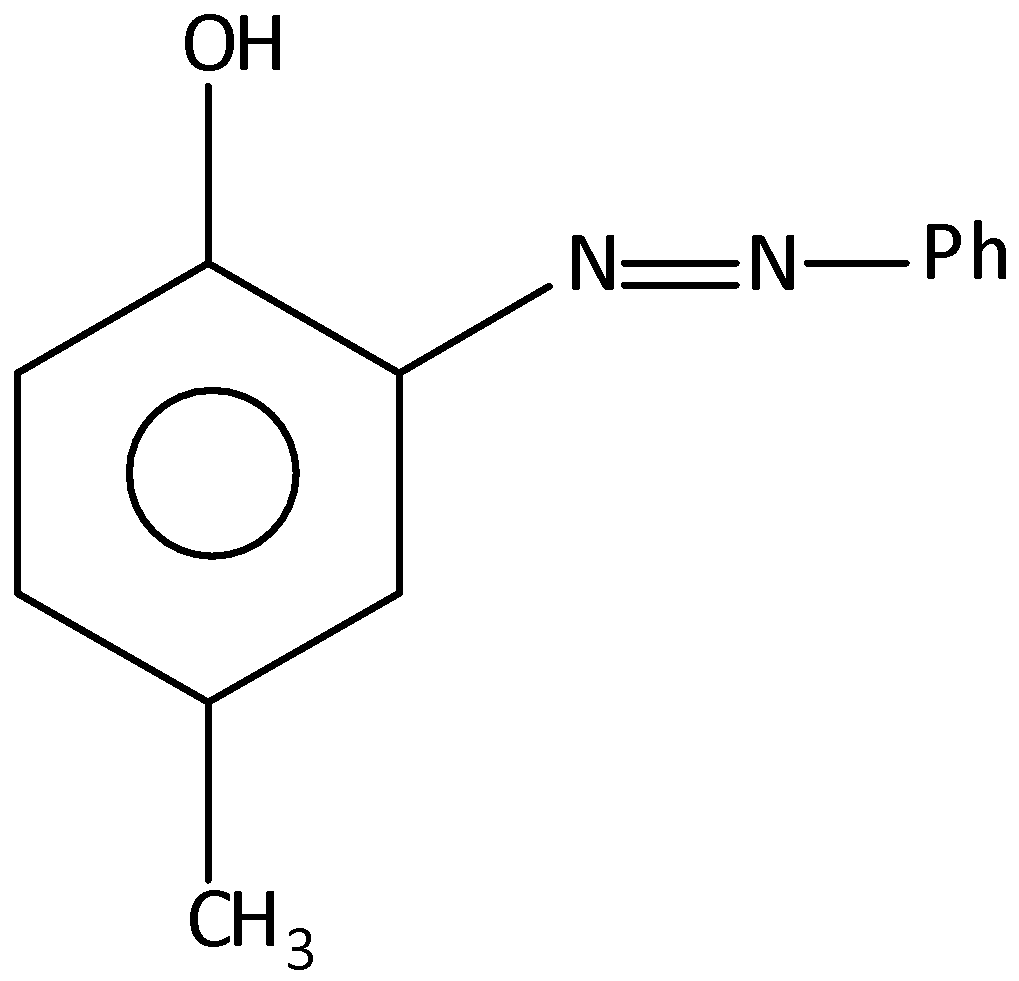
B.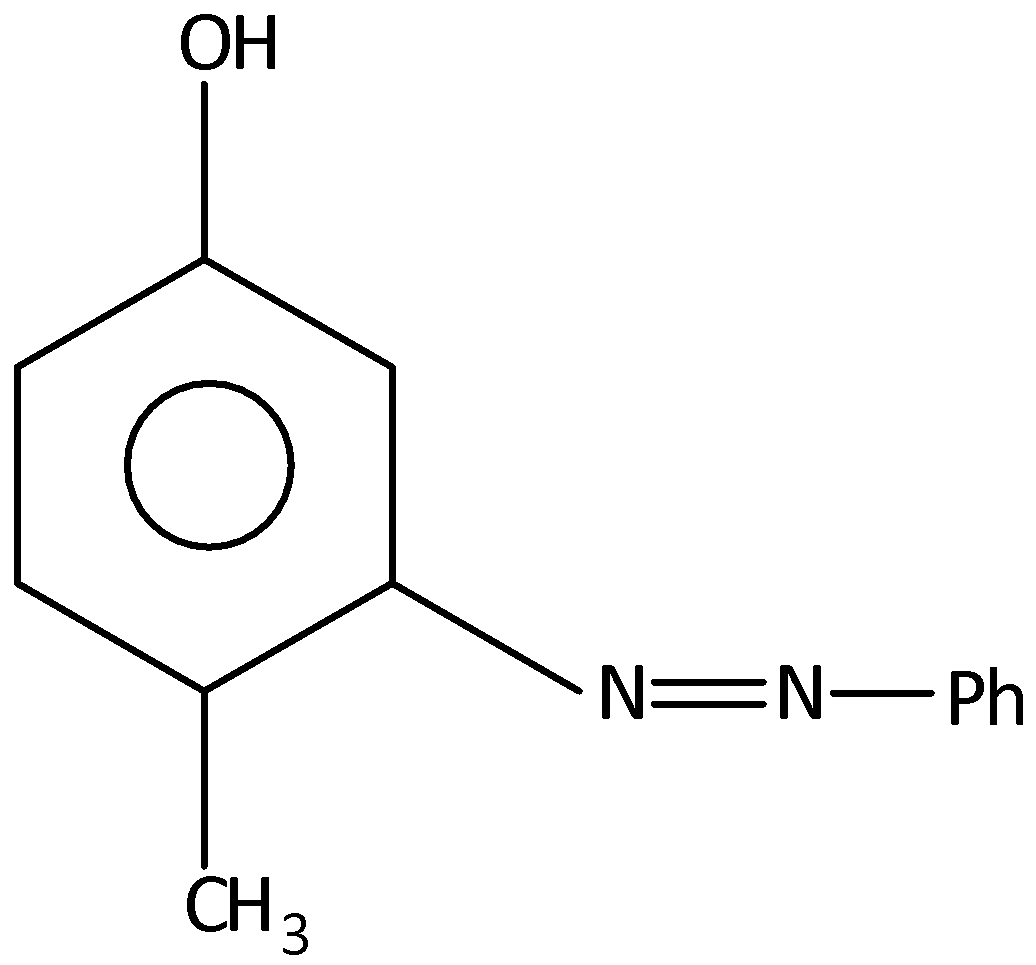
C.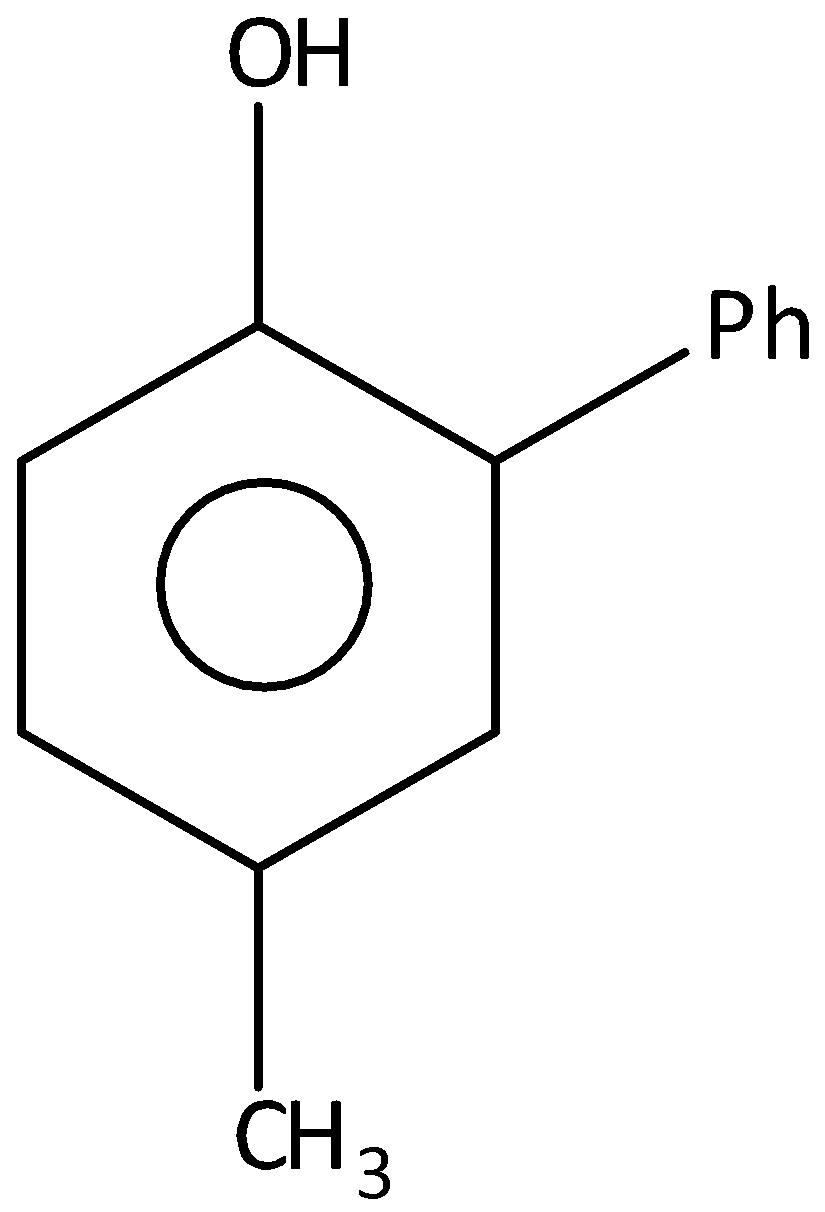
D.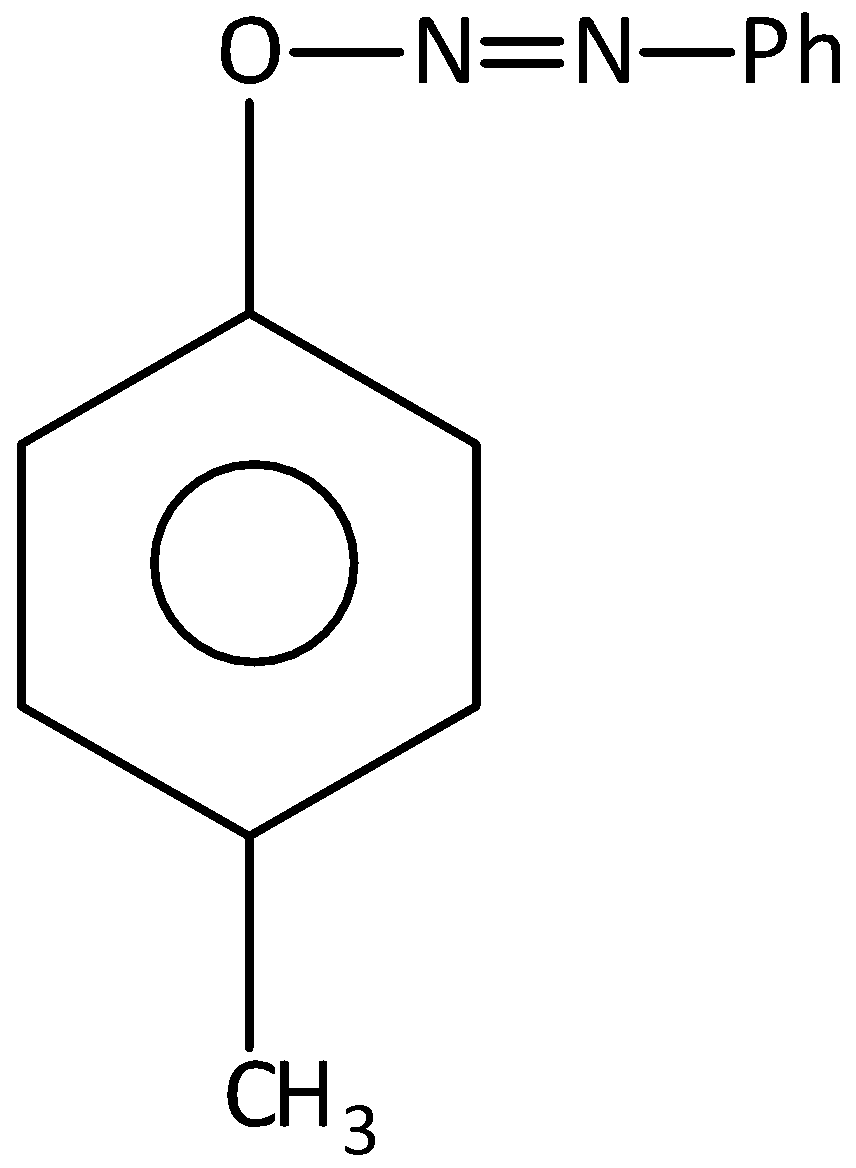
Solution
We have to know that the p-cresol is also known as 4-methyl phenol. It is an organic compound having the formula, CH3C6H4. The p-cresol does not have any colour and it is mainly used as an intermediate while preparing the other chemicals. The p-cresol is a derivative of phenol and it is an isomer of m-cresol and o-cresol. In the case p-cresol, the methyl group is attached on the para position, and a hydroxyl group is directly attached with the phenol ring.
Complete answer:
When an aromatic ring is reacted with benzene diazonium chloride, there is a formation of azo compounds. Here, p-cresol is reacted with benzene diazonium chloride, at the pH range8−10, there will obtain the azo compound, and that is, 4−methyl −2−(phenyldiazenyl) phenol. The benzene diazonium chloride contains the diazonium ions and that is, −N2+group. And this group is attached to the benzene ring. When p-cresol is reacted with benzene diazonium chloride the diazo group will attach to the p-cresol. Let’s see the reaction,
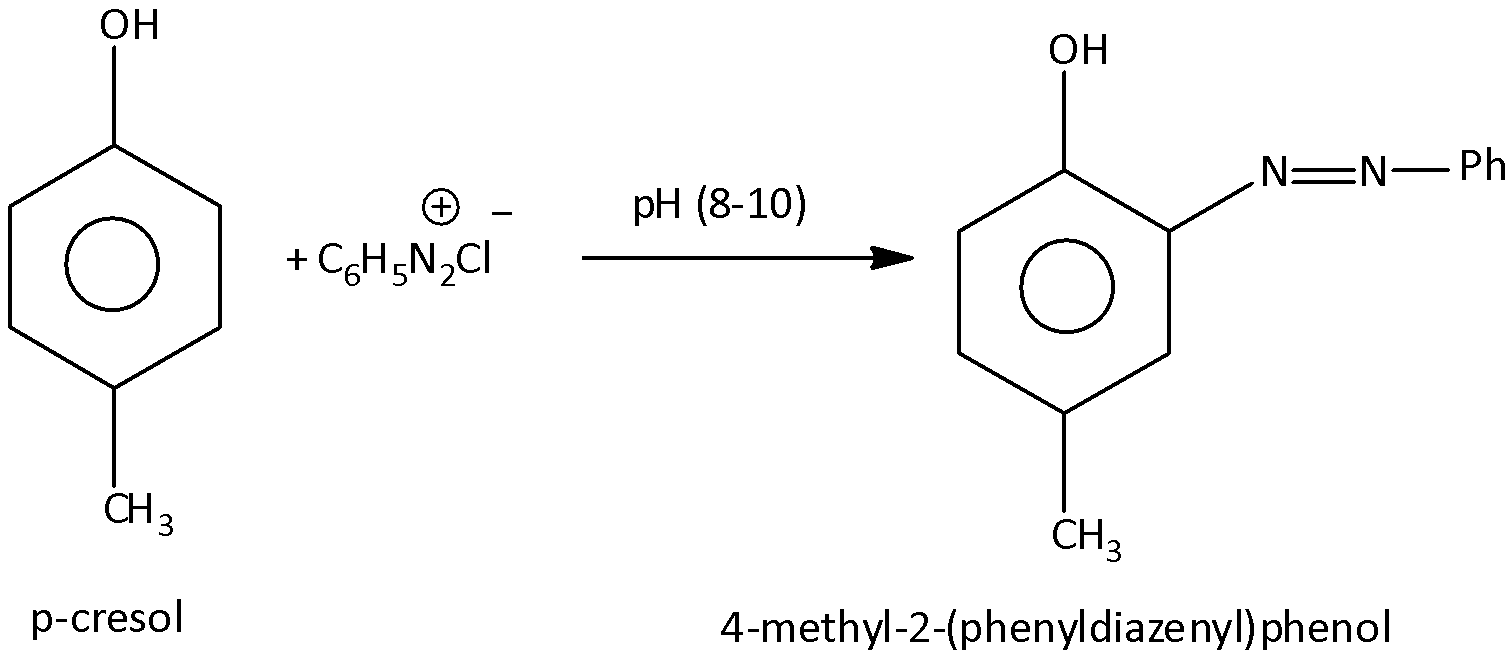
Hence, option (A) is correct.
When p-cresol is reacted with benzene diazonium chloride, there will not be 4−methyl−3−(phenyldiazenyl) phenol. Hence, option (B) is incorrect.
When p-cresol is reacted with benzene diazonium chloride, there will not be 5−methyl-[1,1′−biphenyl]−2− ol. Hence, option (C) is incorrect.
1−Phenyl−2− (p-tolyloxy) diazene is not formed as a product. Hence, option (D) is incorrect.
Hence, option (A) is correct.
Note:
We need to know that when an aromatic compound is reacted with benzene diazonium chloride, there is a formation of azo compound. The azo compound containing the functional group diazenyl, R - N = N - {R^'}. Here, R and R’ may be alkyl or aryl. And the N=Ngroup is known as azo group. And this aromatic azo compound is formed by the reaction of organic substances with diazonium salt.
