Question
Question: What will be the order of reaction for a chemical change having log $t_{1/2}$ vs log a? (where a = i...
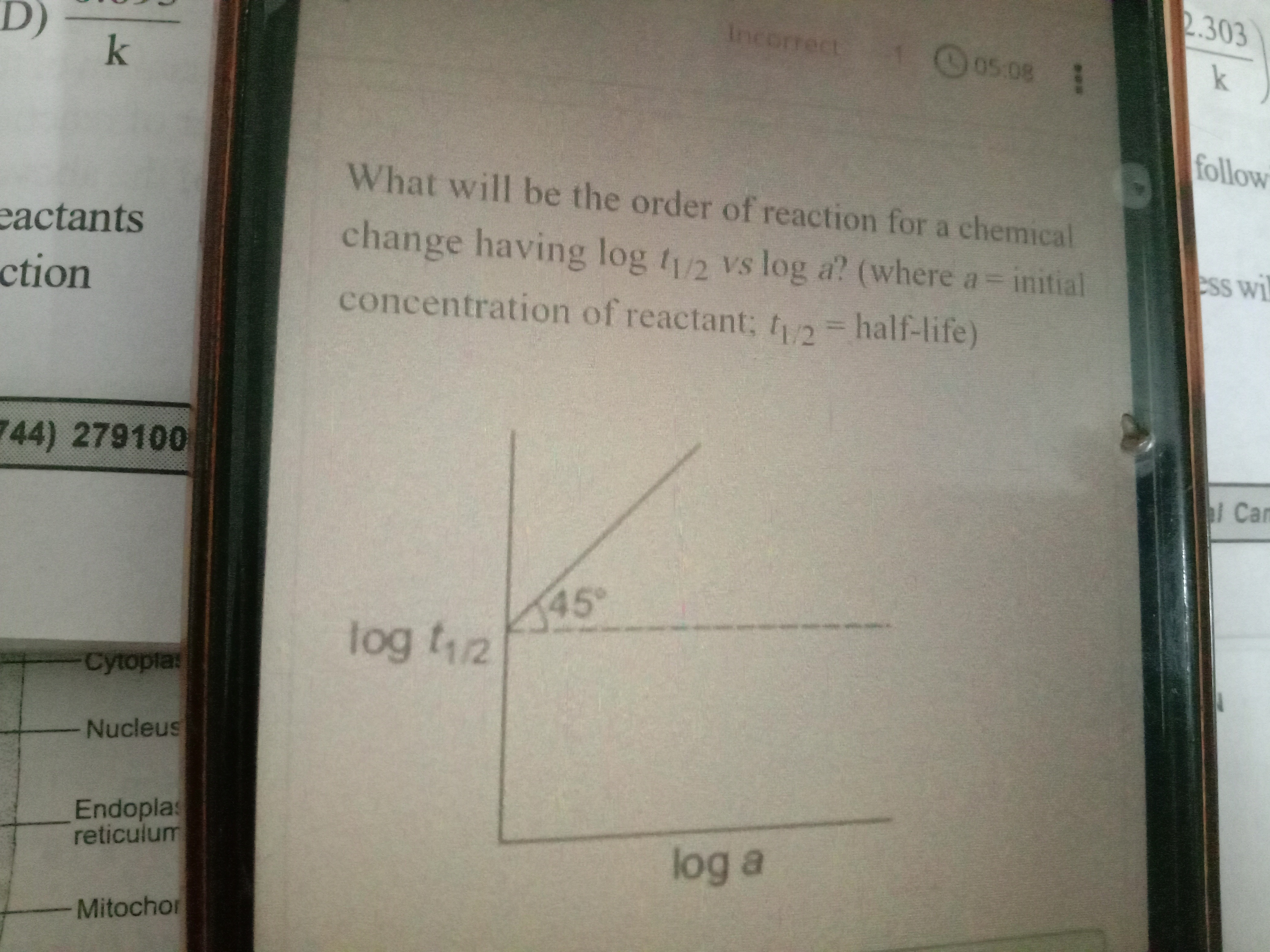
What will be the order of reaction for a chemical change having log t1/2 vs log a? (where a = initial concentration of reactant; t1/2 = half-life)
Answer
0
Explanation
Solution
The half-life (t1/2) for an n-th order reaction is generally proportional to a1−n, where 'a' is the initial concentration. Taking the logarithm of this relationship yields logt1/2=constant+(1−n)loga. This is a linear equation where the slope is (1−n). From the graph, the slope is tan(45∘)=1. Equating the slopes, 1−n=1, which gives n=0.
