Question
Question: What will be the n-factor for \( Ba{(Mn{O_4})_2} \) in an acidic medium? (Where it behaves as oxidan...
What will be the n-factor for Ba(MnO4)2 in an acidic medium? (Where it behaves as oxidant)
(A) 5
(B) 10
(C) 6
(D) 3
Solution
n- Factor is the valency factor or conversion factor of a molecule. n-factor of the substance participating in a redox reaction is equal to the number of moles of electrons lost or gained by the molecule. n – factor of substance in non- redox reaction such as an acid is the number of replaceable hydrogen ions.
Complete answer:
The equation of the given molecule in a redox system where it behaves as an oxidant can be written as the following:
Ba(MnO4)2→Ba2++2MnO4−
Barium being a more electropositive element, it does not get reduced to Ba, thus only MnO4− gets reduced to Mn2+ as it always does in an acidic medium.
Thus that can be represented as:
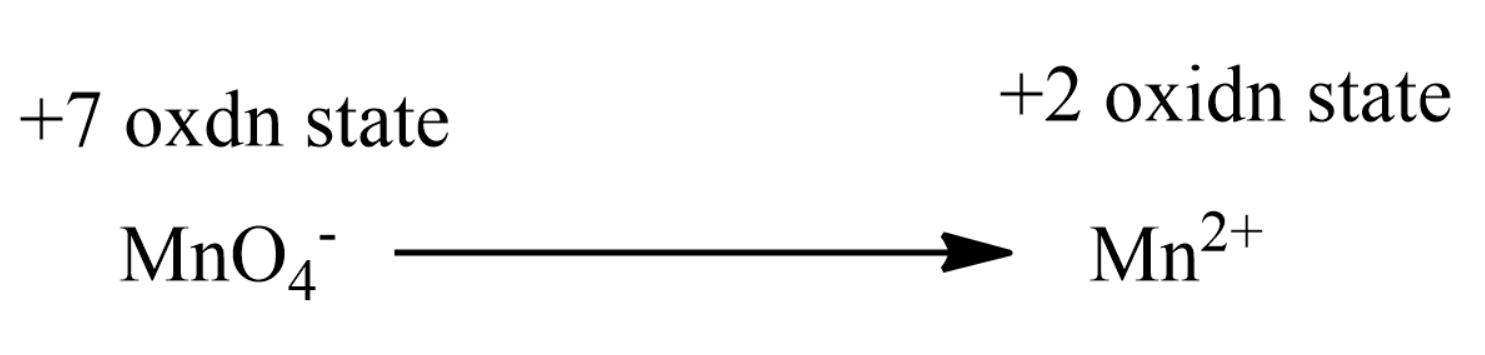
Therefore there is a net change of +5 for each molecule of MnO4−
Since compound has 2 molecules of MnO4− in it so, the n-factor is given by,
(Change in oxidation number per atom or molecule) × (Number of atoms per molecule)
Thus we can say that for this molecule, the n-factor is given as
⇒5×2
⇒10
Thus we can say that the n-factor for Ba(MnO4)2 in an acidic medium will be 10
Thus the correct option is option (2).
Note:
Be familiar with the ways in which we calculate the n-factor of different types of molecules.
For acids the n-factor will be the number of hydronium ions that can be produced.
For bases the n-factor will be the number of hydroxide ions that can be produced.
For redox reactions the n-factor will depend on the change in oxidation state of a given species.
n-factor also depends on the type and extent that the reaction undergoes and hence is not fixed for a given molecule.
