Question
Question: What will be the molecular mass of acetic acid when it is dissolved in benzene?...
What will be the molecular mass of acetic acid when it is dissolved in benzene?
Solution
Benzene is a hydrocarbon i.e. comprises Hydrogen and Carbon molecules. The Acetic acid also has Hydrogen bonds in it. Therefore, it is a nonpolar solvent. Here, the acetic acid undergoes dimerisation via strong H-bond.
Complete answer:
Benzene is an organic chemical compound. Its molecular formula is C6H6. It acts as an organic solvent to acetic acid and is highly soluble in it.
In the Lewis structure of Benzene, we are able to find that the intermolecular forces of the hydrogens are pulling are equally in all directions, so therefore is no net attraction in any direction. This indicates benzene is nonpolar in nature.
Acetic acid is one of the simplest carboxylic acids. Its molecular formula is CH3COOH. The IUPAC name of acetic acid is Ethanoic acid.
Ethanoic acid being highly soluble in benzene solvent, dimerises due to the development of strong hydrogen bonding between its molecules.They have very high Boiling points and possess intermolecular dipole-dipole interaction too.
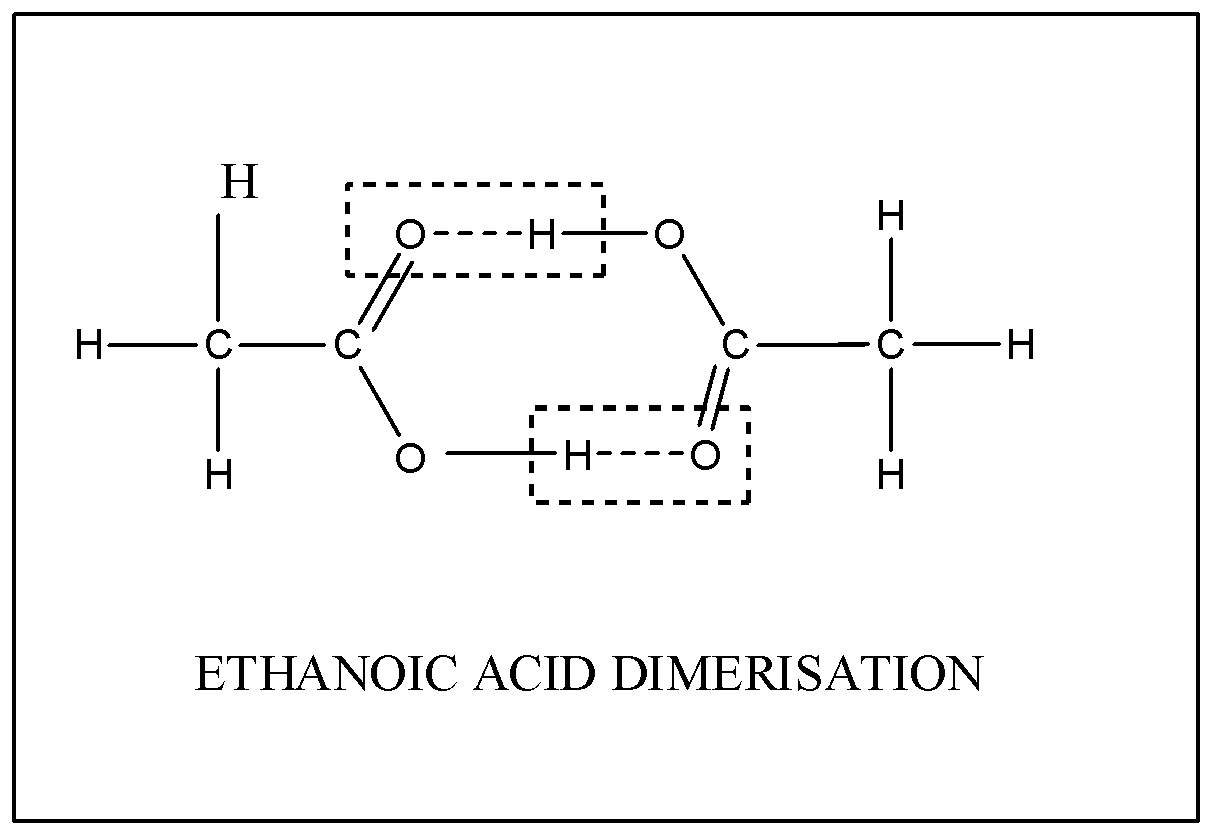
Hence, the molecular mass of Ethanoic acid changes from its normal molecular mass
[Molecular mass of Ethanoic acid = ( 2) = 60 g/mol.] is 60 g/mol.] is 60 g/mol.
[ ∵Molecular weight of Carbon(C) = 12g/mol, Molecular weight of Oxygen(O) =16 g/mol, Molecular weight of Hydrogen(H) =1 g/mol.]
So, after dimerization of Ethanoic acid within Benzene solvent, the molecular mass gets doubled i.e. [ Molecular mass of Ethanoic acid after Dimerization= 60 g/mol×2=120g/mol. ] 120 g/mol.
The molecular mass of Acetic acid (Ethanoic acid) is 120g/mol when it is dissolved in Benzene.
Note: Do not forget to mention the dimerization process occurring when acetic acid is dissolved in benzene. Many make mistakes by writing the answer as 60g/mol since this is the normal molecular mass of Acetic acid or Ethanoic acid.
