Question
Question: What will be the major product in the following reaction? 
(A) 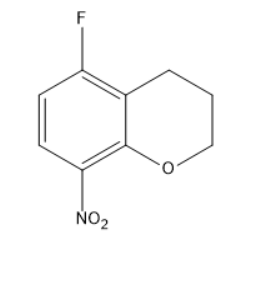
(B) 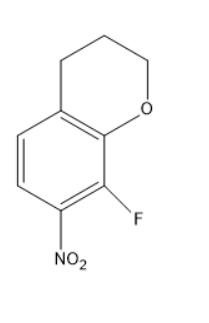
(C) 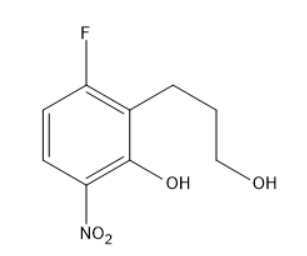
(D) 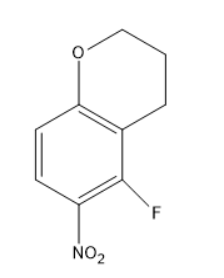
Solution
Aromatic compounds undergo substitution reactions easily. They are two types of substitution reactions namely electrophilic substitution and nucleophilic substitution reactions. When compared to ortho and para positions, the substitution takes place at para position only due to less energy.
Complete answer:
Given compound has one nitro group, two fluorine atoms, one hydroxyl group. When the given compound is treated with a base like potassium hydroxide, undergoes dehydrohalogenation i.e. elimination of molecules like HF .
In the given compound, the elimination of hydrogen atom from alcohol and fluorine atom from the para position will be removed but not ortho position. This is due to the fact that ortho substitution requires more energy when compared to the para substitution product.
Thus, the fluorine atom which is para to the nitro group was substituted by the alkoxide group. The chemical reaction will be as follows:
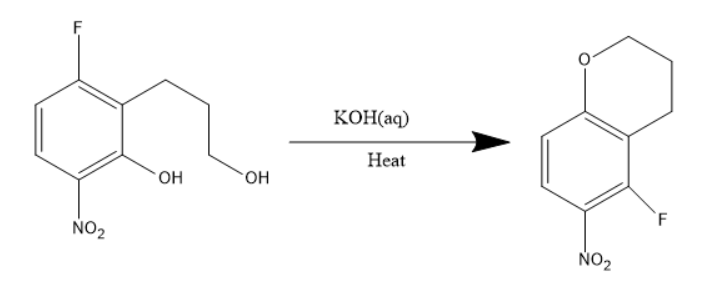
The above product is given in option D.
Option A consists of an ortho-substituted alkoxide product, which requires more energy and is not the major product.
Option B does not consist of an oxygen atom in the proper arrangement.
Option C has the substitution of nucleophile in place of fluorine only, but does not undergo cyclization.
Thus, option D is the correct one.
Note:
Aromatic compounds are stable compounds as they consist of conjugation of pi-electrons, cyclic, planar, and obeying Huckel’s rule. Aromatic compounds undergo only substitution reactions but not addition reactions. As these compounds cannot lose their aromatic character.
