Question
Question: What will be the major product in the following mononitration reaction? 
A.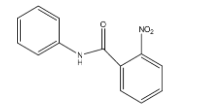
B.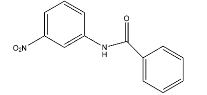
C.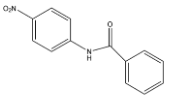
D.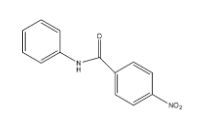
Solution
The addition of NO2+ is called nitration. NO2+ is an electrophile which means electron loving or electron deficient group. Nitro group is going to attach to the carbon where there is a presence of a high number of electrons.
Complete step by step answer:
- In the question it is asked if we are going to mononitrate to the given molecule in the reactant side, where the nitro group is going to attack.
- The given reactant is as follows.
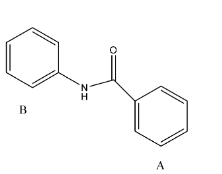
- In the above molecule there are two rings labeled as A and B.
- Whenever we are going to do the nitration in the given molecule there is a possibility to attack on both the rings A and B because both A and B rings benzene rings.
- Coming to Ring A. The ring A is attached to the carbonyl group. We know that the carbonyl functional group is going to act as an electron withdrawing group.
- So the ring A is becoming electron deficient due to the attachment of a carbonyl functional group. So, mononitration is not possible in ring-A.
- Coming to ring B. Ring B is attached to nitrogen (amine group).
- We know that due to the presence of lone pair of electrons on the nitrogen they are going to activate the ring B-
- Therefore the nitration is going to occur in ring B at para position due to the presence of steric hindrance at ortho position.
- The mononitration of the given molecule is as follows.
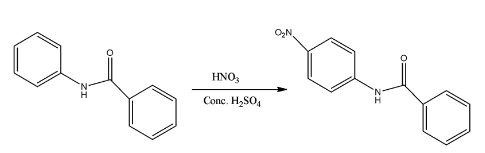
Therefore the correct option is C.
Note: Nitro group is an electron loving group that is why it is going to attach to the carbon where there should be the presence of the huge amount of electrons. Amino group is an ortho and para directing group when it is attached to a benzene ring.
