Question
Question: What will be the heat absorbed if the work done on the system along the curved path ‘ba’ is 52 J. ...
What will be the heat absorbed if the work done on the system along the curved path ‘ba’ is 52 J.
A.-140 J
B.-172 J
C.140 J
D.172 J
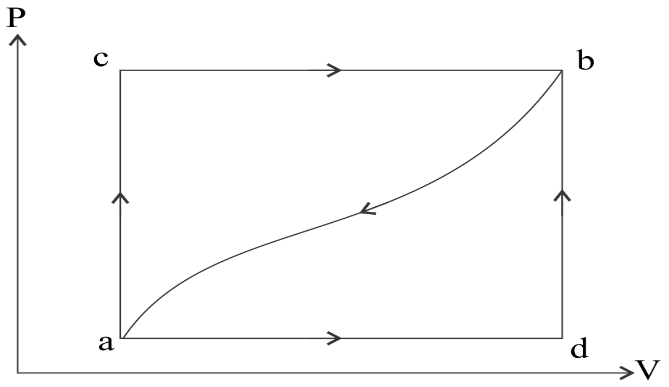
Solution
Every point on a PV diagram represents a different state for the gas (one for every possible volume and pressure). As a gas goes through a thermodynamics process, the state of the gas will shift around in the PV diagram, tracing out a path as it moves. Being able to decode the information shown in a PV diagram allows us to make statements about the change in internal energy ΔU, heat transferred Q, and work done W on a gas.
Complete answer:
-If we press the piston downwards, the volume of the gas will decrease, so the state must shift to the left toward smaller volumes. Since the gas is being compressed we can also say for sure that positive work W is being done on the gas.
-Similarly, if we let the gas expand, pushing the piston upward, the volume of the gas will increase, so the state must shift to the right toward larger volumes. Since the gas is expanding we can also say for sure that negative work W is being done on the gas.
-The work done during a thermodynamic process is equal to the area under the curve.
Internal energy and temperature are proportional. So if the temperature increases, the internal energy must also increase.
-We know that,
ΔU=Q−W here,ΔU=−120 and W=−52J
Substituting the values
Q=ΔU−W Q=−120−52 Q=−172J
Hence, the correct answer is (B).
Note:
Internal energy and temperature are proportional. So if the temperature increases, the internal energy must also increase. So if the quantity of pressure times volume increases, the temperature T and internal energy U must also increase.
