Question
Question: What will be the formula and electron-dot structure of cyclopentane?...
What will be the formula and electron-dot structure of cyclopentane?
Solution
Along with the straight chain compounds, we can also form ring-like structures. These can even have double bonds in them. Cyclopentane is a cyclic saturated compound. It has five members in the ring. It has two hydrogen atoms less than its parent alkane-pentane.
Complete step by step answer:
Hydrocarbons are the compounds containing carbon and hydrogen. There are two main types-saturated hydrocarbons and unsaturated hydrocarbons. A saturated hydrocarbon has only a carbon-carbon single bond. Unsaturated hydrocarbons have double or triple bonds in them.
Saturated hydrocarbons constitute a family of straight or branched chain compounds called alkanes. They have carbon-carbon single bonds and only carbon and hydrogen. The first member of the alkane series is methane. It has a carbon atom and three hydrogen are attached to carbon by a single bond. It can be represented as CH4 . Now the alkane containing two carbon atoms is ethane. Its molecular formula is C2H6 . So the members of alkane family can be shown as-
| Alkane | Structure or formula |
|---|---|
| Methane | 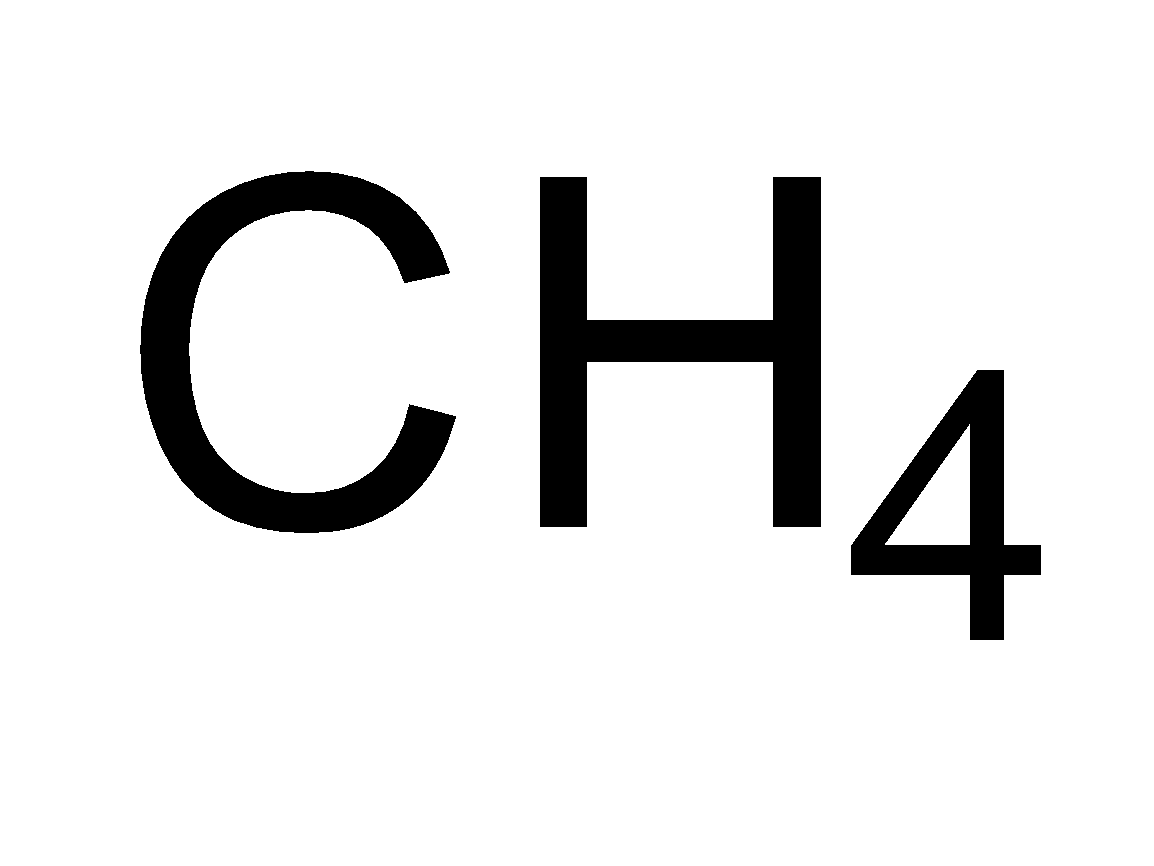 |
| Ethane | 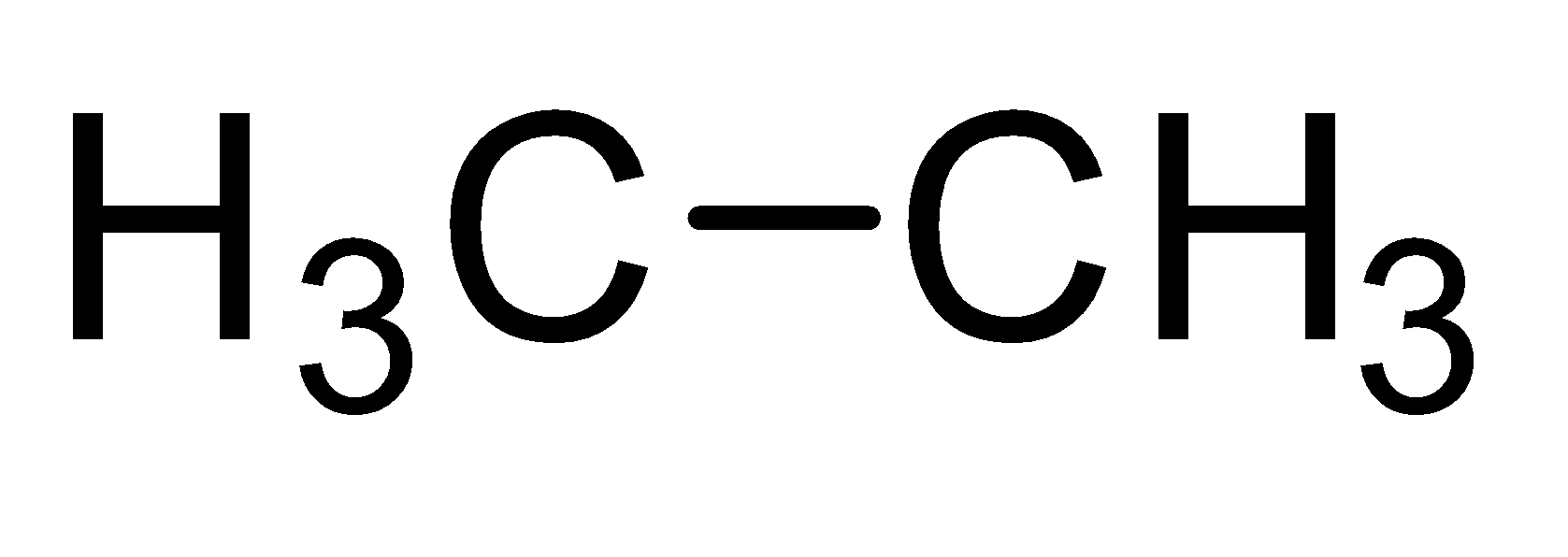 |
| Propane | 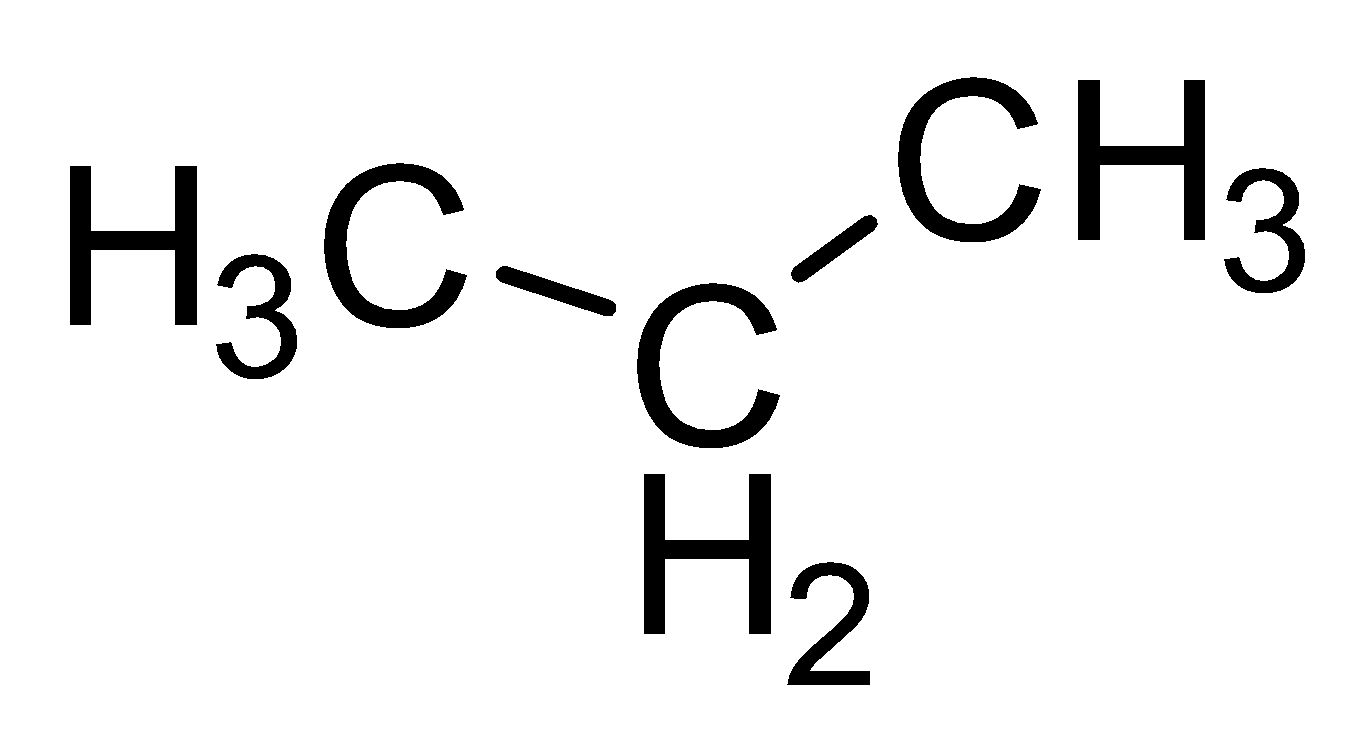 |
| Butane | 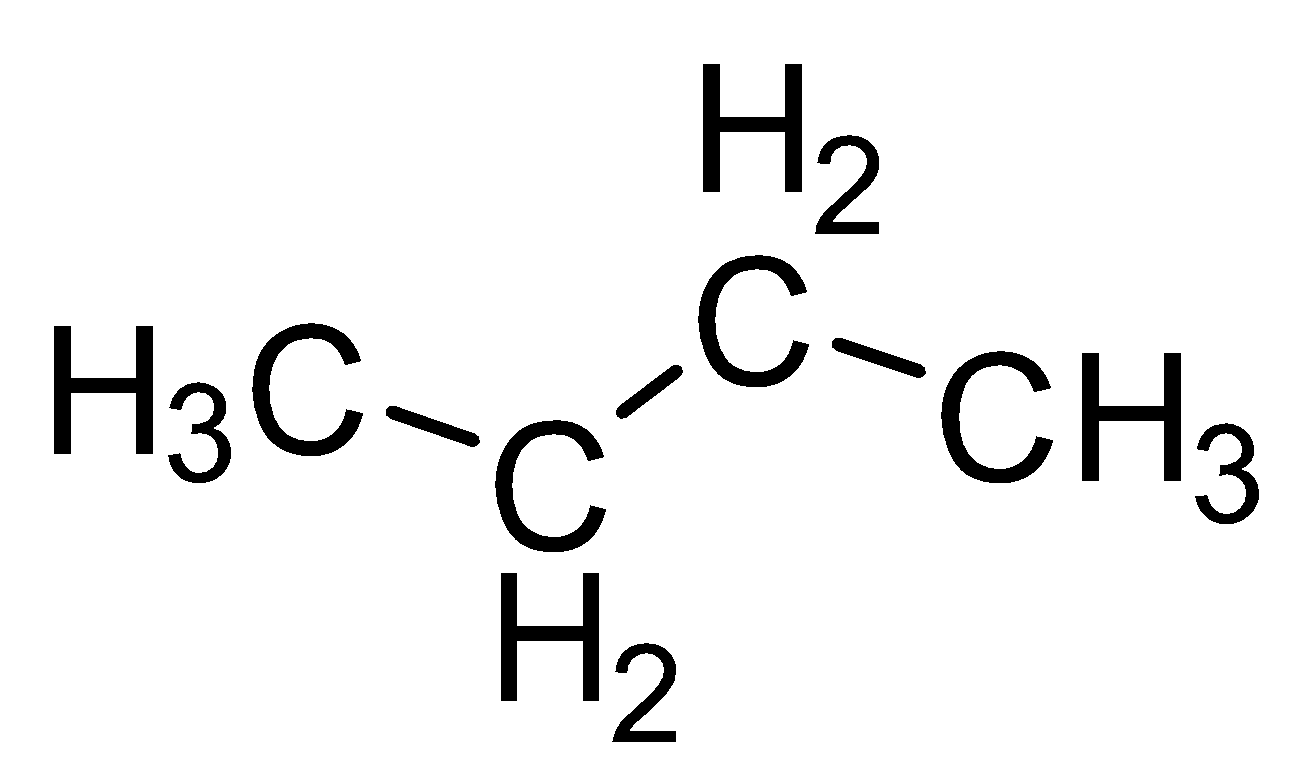 |
| pentane | 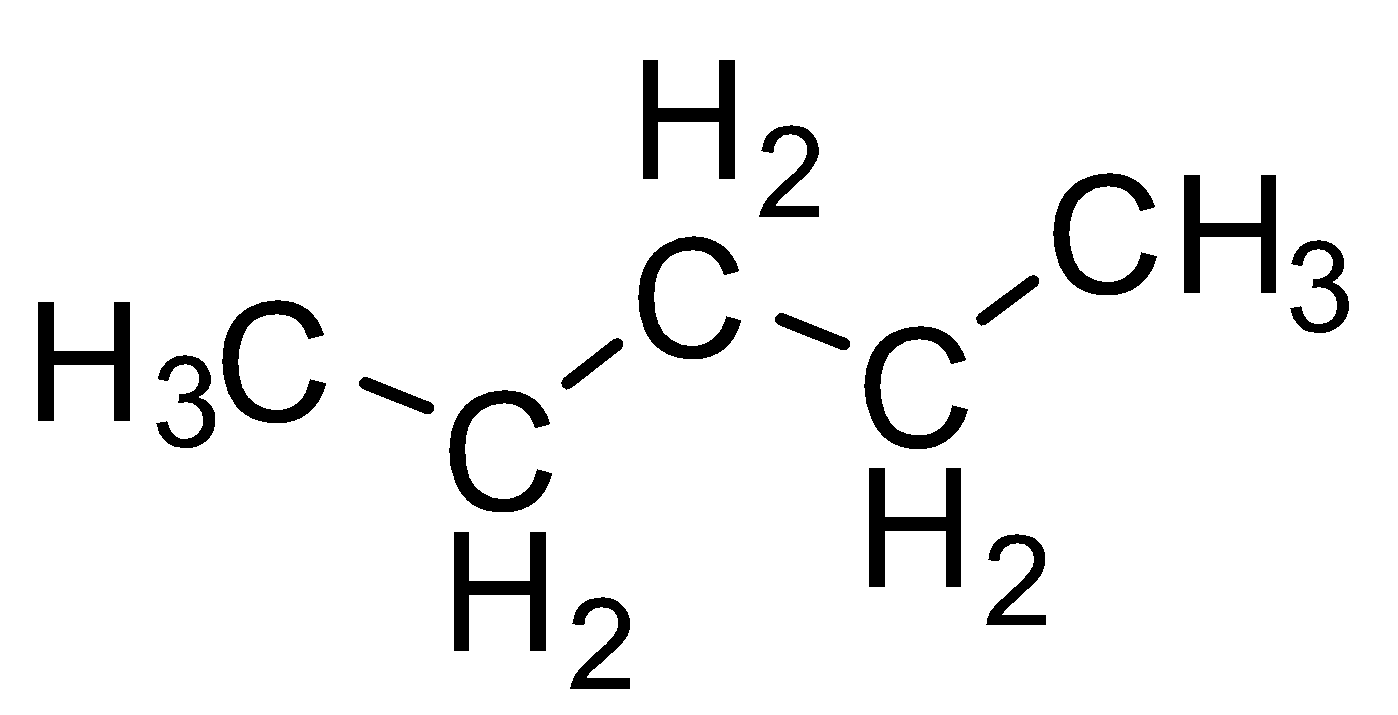 |
The names of members of the alkane family end with ‘–ane’.
Now let us talk about the cyclic compounds. In cyclic compounds atoms are connected to each other and form a ring-like structure. Cyclopentane can be formed from pentane by joining end carbon atoms. The structure of cyclopentane will be-
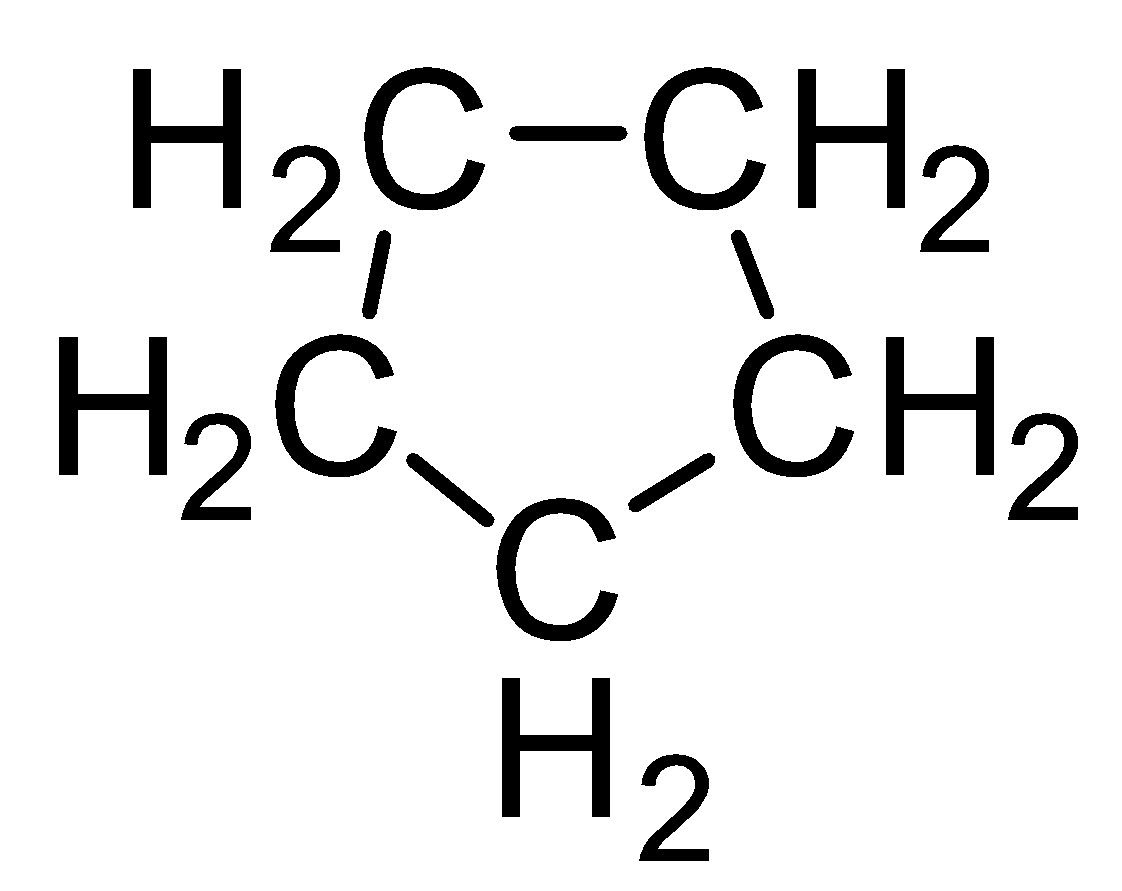
In this structure there are five groups of atoms connected to each other by a single carbon-carbon bond. The formula of cyclopentane can be determined from the above structure too. It is given as C5H10 .
In the electron-dot structure, the valence electrons of atoms are represented around them as dots. The number of dots is equal to the valence electrons in an atom. The electron-dot structure of cyclopentane is-
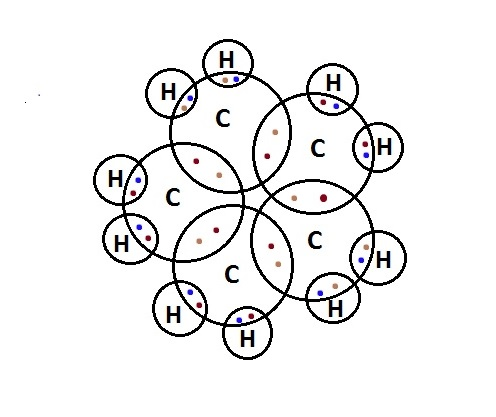
So, the correct answer is “Option A”.
Note: Remember we show only valence electrons in the electron-dot structure of a compound. Cyclopentane has two hydrogen atoms less than straight chain compound pentane. These two hydrogen atoms are lost during the formation of a ring.
