Question
Question: What will be the final product of the reaction? 
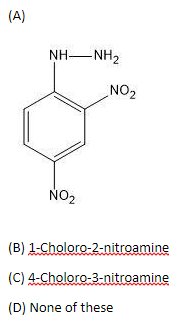
Solution
A good nucleophile can stabilize the reaction and also it makes it easy to replace the existing substitute. Here, the nucleophile is hydrazine so the product will have the substitute which would be related to the hydrazine molecule.
Complete step by step solution:
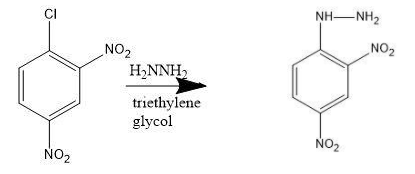
Let us discuss the process of nucleophilic addition of hydrazine: The given reaction is of 2, 4 dinitrochlorobenzene reacting with hydrazine. The presence of two nitro groups which are electron accepting groups, makes it easy for chloride to get displaced. Chlorine is the ortho, para directing group. So, nitration of chlorine is feasible for stable products. Chlorobenzene is prepared from benzene (here the reactant).
Procedure-
-2, 4 dinitrochlorobenzene is dissolved in triethylene glycol. The hydrazine solution is added to it.
-The mixture is then refluxed by stirring it for an hour nearly.
-The product starts forming in the prior ten minutes only.
-It is then cooled and again alcohol is added to remove unchanged halide.
Illustration-
-The reaction of 2, 4 dinitrochlorobenzene with hydrazine in the presence of triethylene glycol gives nucleophilic substitution reaction. This forms the stable product as 2, 4 dinitrophenylhydrazine.
-Reaction takes place as follows;
Therefore, option (A) is correct.
Additional information:
The aqueous solution of 2, 4 dinitrophenylhydrazine (DNP) is known as Brady’s reagent which reacts with carbonyl compounds to give coloured precipitates who have a sharp melting point.
DNP has the risk of explosion, so it's transported wet.
Note: The good nucleophile eases the reaction and hence replacement of chloride is feasible when hydrazine takes the position. If any of the options have hydrazine substitute, then you can ignore option (D) from the very beginning of the solution.
