Question
Question: What will be the E cell of the cell given: \( Pt\,|{H_2}\,(1\,atm)\,|\,{H^ + }\,(0.001M)\,|\,{H^ ...
What will be the E cell of the cell given:
Pt∣H2(1atm)∣H+(0.001M)∣H+(O.1M)∣H2(1atm)∣Pt
(a) 0.1182V
(b) −0.1182V
(c) 0.0591V
(d) −0.0591V
Solution
Hint : Nernst equation is used to calculate the cell potential of an electrochemical cell at a given pressure, temperature and concentration of the reactant. The value of the cell potential of standard hydrogen electrodes at temperature 298K i.e. E0cell is taken as zero.
Complete Step By Step Answer:
The above equation is the redox reaction of hydrogen gas and the platinum electrode acts as a reference electrode. Platinum electrode is inert in nature so it cannot corrode easily. It also improves reaction kinetics by absorbing hydrogen at its interface.
We know that standard hydrogen electrode (SHE) is used as a reference for comparison with any other electrode and it is measured at temperature 298K and the pressure of hydrogen gas is equal to 1 Bar. This reaction takes place on platinum electrode The value of the cell potential for SHE is declared as zero and called as standard hydrogen electrode potential i.e. E0cell .
Reaction at Anode i.e. oxidation
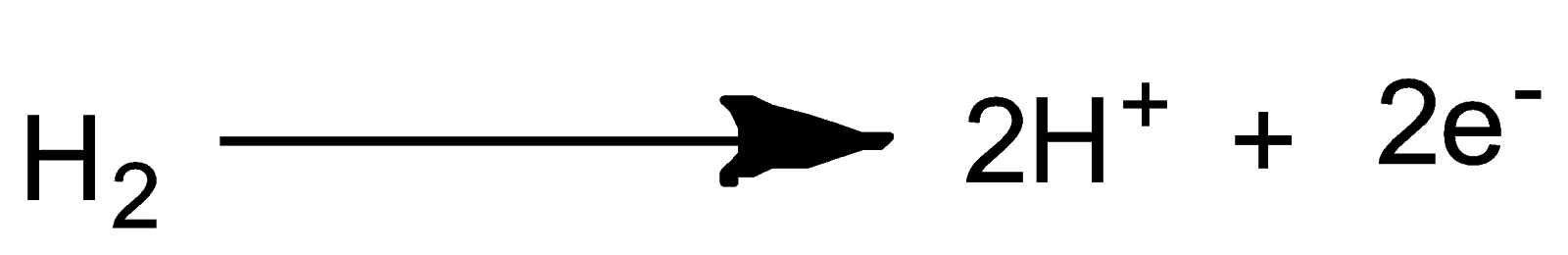
Reaction at Cathode i.e. reduction
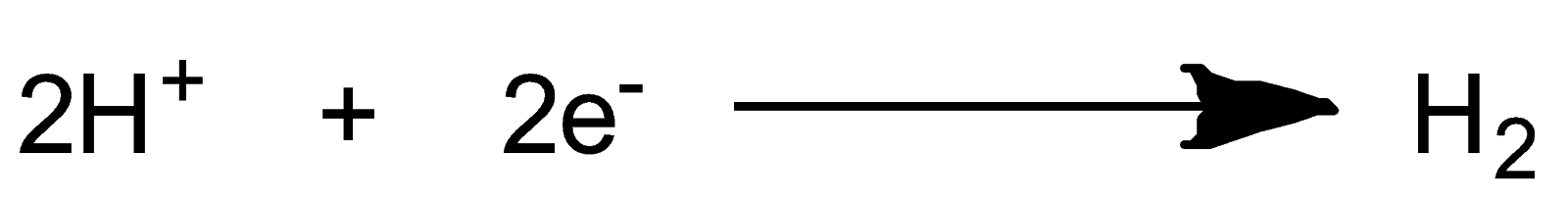
The complete chemical reaction is as follow:-
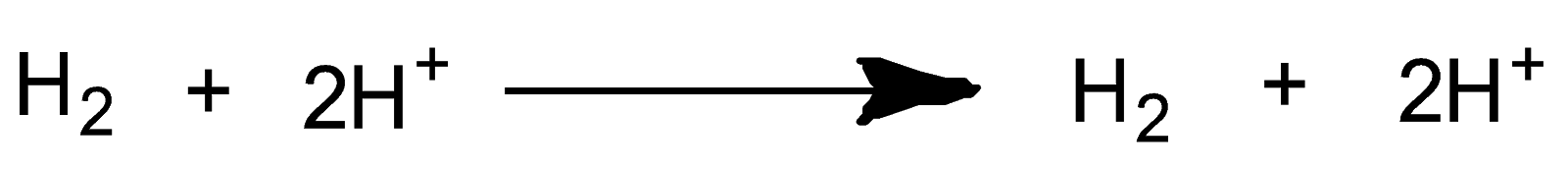
According to the Nernst equation-
Ecell=E0cell−nF2.303RTlogKeq
Where,
E0cell =Standard electrode potential
E cell = Electrode potential
R= Universal Gas Constant ( 8.314JK−1mol−1 )
T= temperature in Kelvins
F= Faraday’s constant ( 96500C/mol )
Keq =Equilibrium constant
n= number of electrons transferred in the cell.
By substituting values of R, T ( 298K ),and F
We get,
Ecell=E0cell−n0.0591logKeq
Here, in this question the pressure of H2 is the same, so we do not consider it.
Ecell=E0cell−n0.0591log[2H+][H+]
We know that,
E0cell=0.0V
Ecell=−n0.0591log(0.1)(0.001)
Here, n= 2
Ecell=−20.0591(−2)log10
Ecell=−20.0591(−2)
We get,
E cell = 0.0591V
The E cell of the given cell is 0.0591V
Hence, the correct answer is option (C).
Note :
Always remember that the Platinum is considered as an inert electrode so it does not participate in the redox reaction but it provides oxidation and reduction surface. The value of standard hydrogen potential (SHE) is taken as zero. Also, the Nernst equation is derived from the Gibbs free energy at standard conditions, but it enables the determination of cell potential under non- standard conditions. It also allows the determination of equilibrium constants as it relates the measured cell potential to the reaction quotient.
