Question
Question: What will be S : 
A. 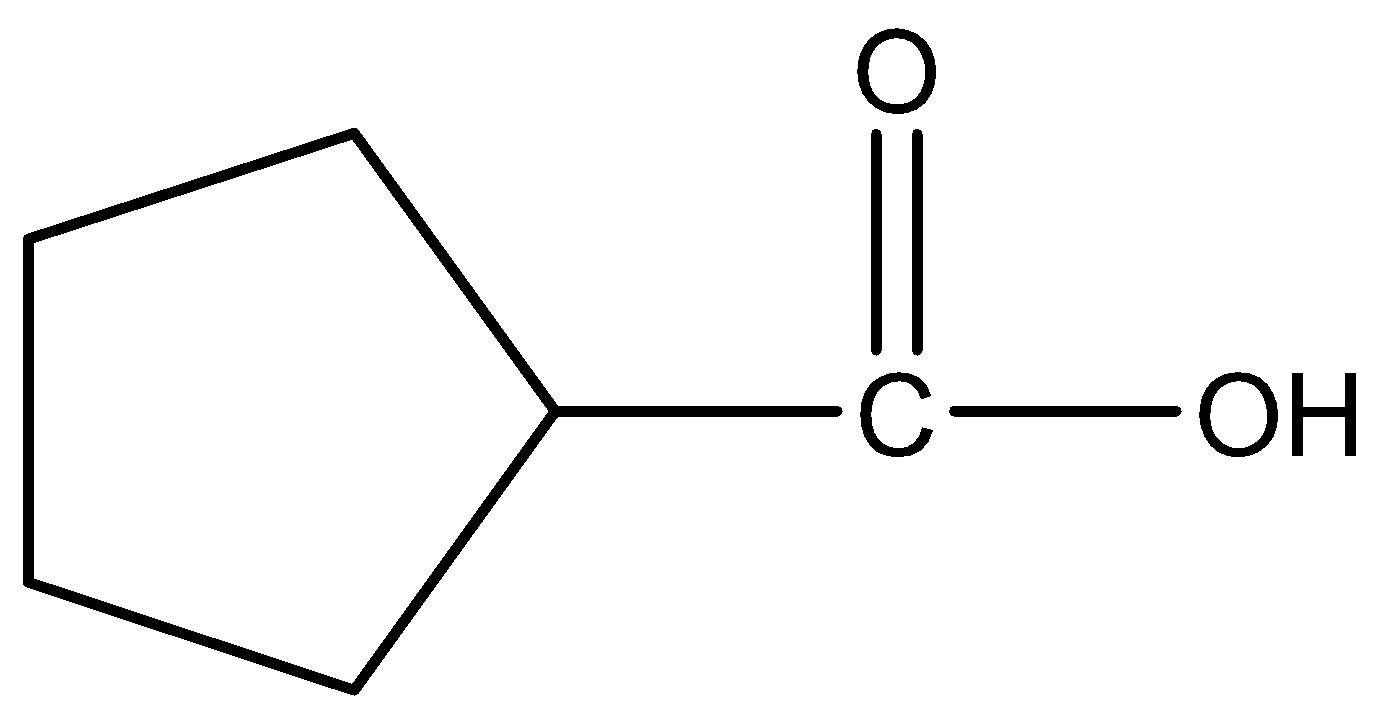
B. 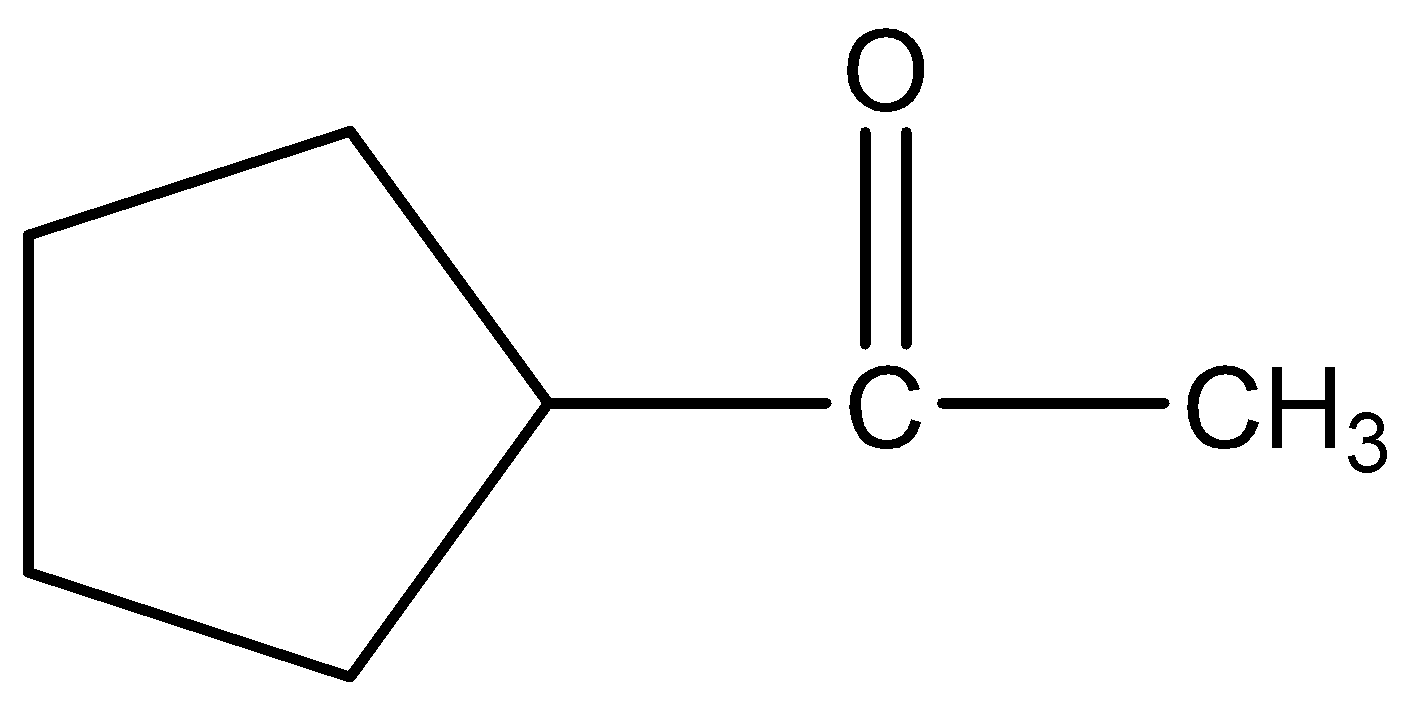
Solution
When an amide is treated with dehydrating agents it gets converted into nitriles. Nitriles will get converted to imines in the presence of Grignard reagents and imines on treatment with aqueous acid (H3O) to give ketones. When a methyl ketone is treated with base and a halogen such as I2, Br2, or Cl2, it is converted into a carboxylic acid, along with a haloform (HCX3)
Complete step by step answer:
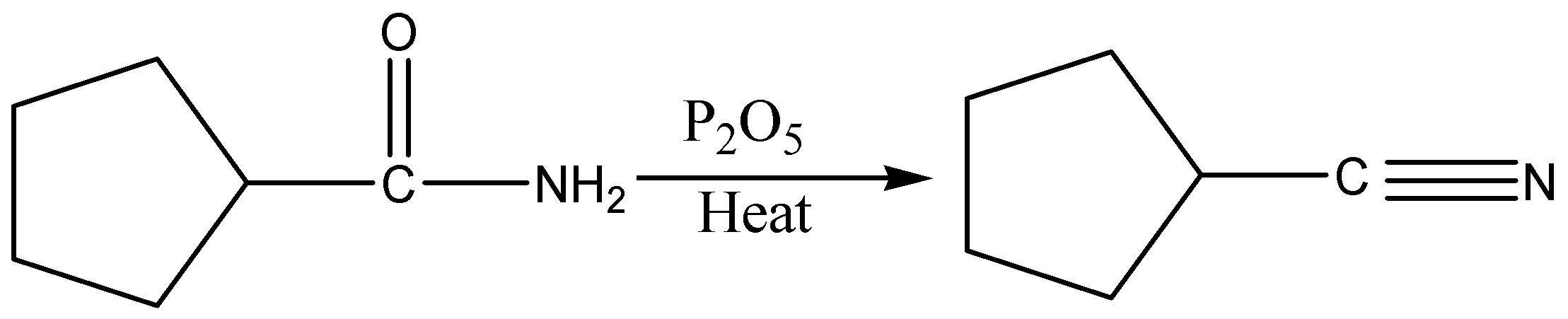
When an amide is treated with dehydrating agents it gets converted into nitriles.
In the given case, P2O5 is a dehydrating agent. When they are heated, amide gets converted into nitriles. Phosphorus pentoxide (P2O5) has a strong affinity for water and therefore, acts as a powerful dehydrating agent.
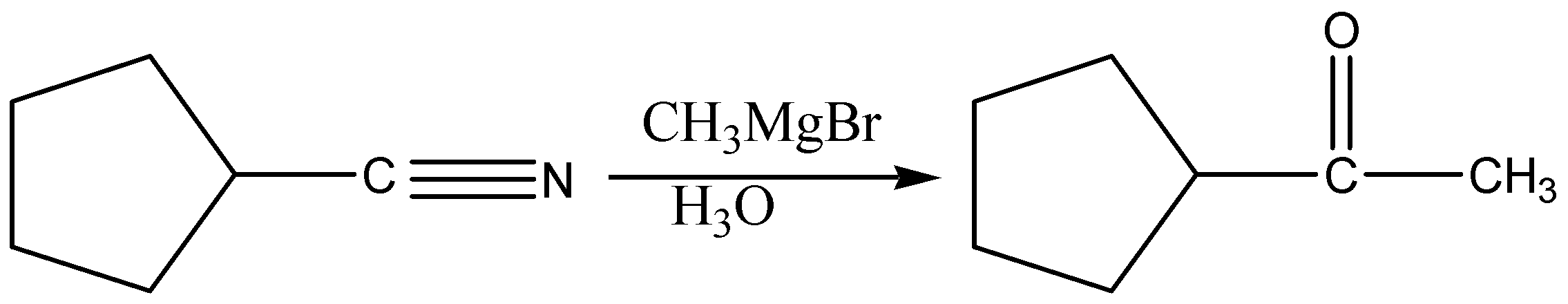
Grignard reagents (CH3MgBr) will add once to nitriles to form imines. The imines can be treated with aqueous acid (H3O) to give ketones.
When a methyl ketone is treated with base and a halogen such as I2, Br2, or Cl2, it is converted into a carboxylic acid, along with a haloform (HCX3).
The reaction proceeds through three successive cycles of deprotonation and halogenation at the alpha carbon, followed by addition of base to the carbonyl and expulsion of CX3 as a leaving group.
The final breakage of the C−C bond only happens with methyl ketones, however! Alkyl ketones with alpha C−H bonds just give products from alpha-halogenation.
Since a C−C bond is being exchanged for a C−O bond, this is a net oxidation – indeed, one of the few methods we learn in introductory organic chemistry for the oxidation of a ketone.
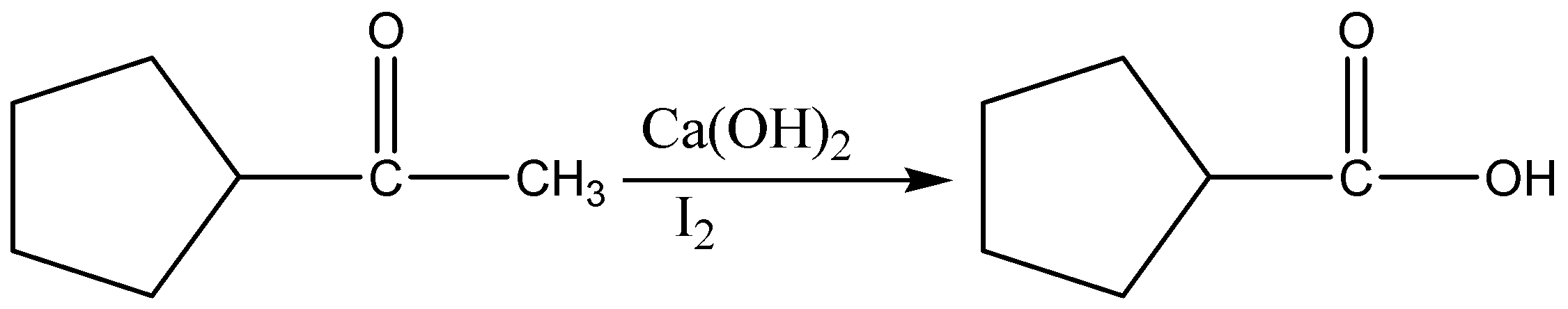
Therefore, the correct answer is option (A).
Note: The Grignard reagent adds to the carbon of the nitrile, forming a new carbon-carbon bond. This is stable until water and acid is added which forms the imine. Protonation of the imine nitrogen results in the formation of the iminium ion, which undergoes 1,2-addition by water. This species then undergoes proton transfer to allow for the loss of ammonia (NH3) in a subsequent 1,2-elimination. Deprotonation of the carbonyl oxygen then results in the ketone.
