Question
Question: What will be compound \(A\) in the following reaction? 
A.) 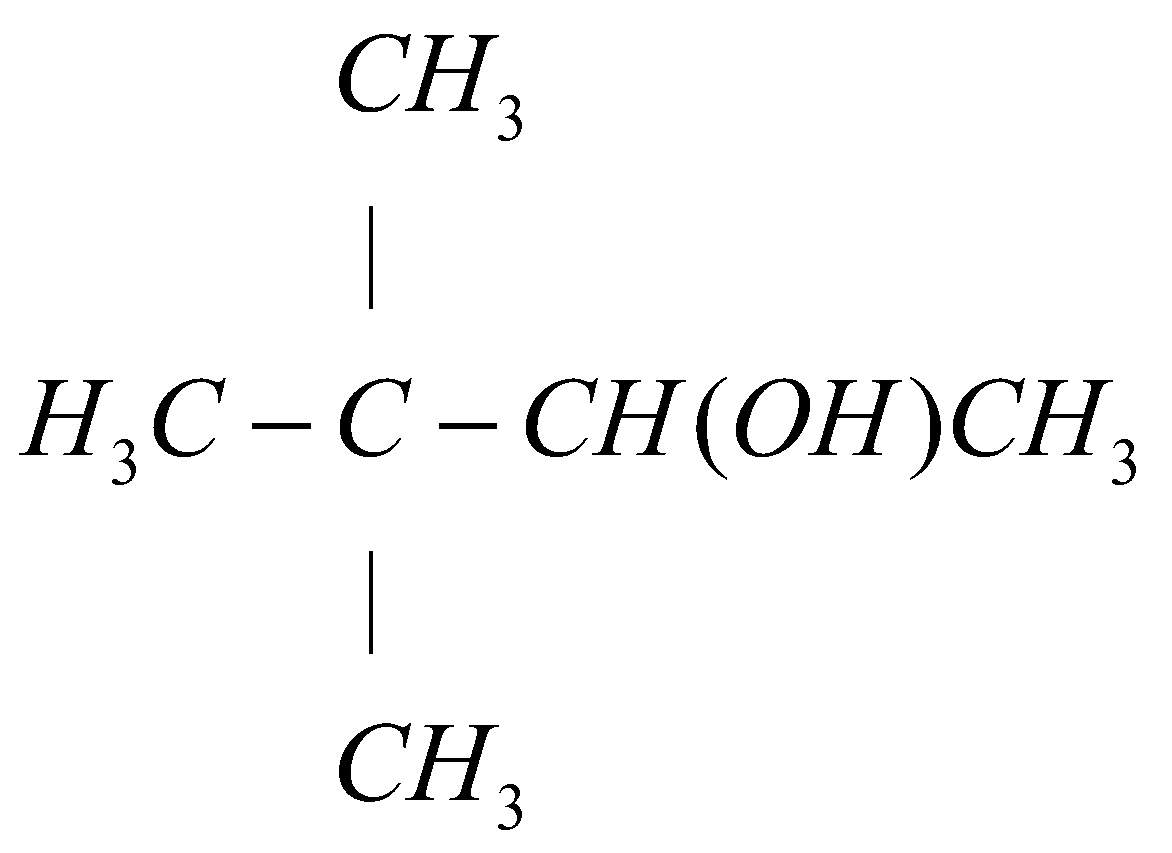
B.)
C.)
D.) 
Solution
In this question, this reaction happens by the mechanism of oxymercuration-demercuration mechanism in which the hydroxyl group that is OH attaches to the most substituted carbon and H attaches to the least substituted carbon.
Complete step by step solution:
In this given reaction the regents given that are Hg(OAc)/THF and NaBH4/NaOH are the reagents of oxymercuration-demercuration reaction. Here, in these reagents Ac represents acetone. This oxymercuration- demercuration reaction is a two step pathway that is used to produce corresponding alcohol by the hydration of the alkene. That is by reacting alkene with reagents of oxymercuration- demercuration reaction we can get corresponding alcohol. The oxymercuration- demercuration reaction follows the Markovnikov regioselectivity rule. According to Markovnikov regioselectivity rule, the OH group will attach to the most substituted carbon and the H (Hydrogen) will attach to the least substituted carbon.
In the given alkene,
The carbon number 1 of alkene functional group is least substituted so the hydrogen (H) will attach with the carbon number 1 and the carbon number 2 of alkene group is more substituted so hydroxyl group (OH) will attach with carbon number 2. Hence the final reaction can be represented as:
CH3 | H3C−C−CH=CH2 (i)Hg(OOCCH3)2/THF(ii)NaBH4,NaOH | CH3 CH3 | H3C−C−CH(OH)CH3 | CH3
Hence, option (A) is the correct answer.
Note: Always remember that the oxymercuration- demercuration reaction mechanism follows the Markovnikov regioselectivity rule and when alkene reacts with these reagents of oxymercuration demercuration mechanism then the product formed is a corresponding alcohol.
