Question
Question: What type of isomers are \[C{H_3}C{H_2}OC{H_3}\] and \[C{H_3}C{H_2}C{H_2}OH\]? A) Functional B) ...
What type of isomers are CH3CH2OCH3 and CH3CH2CH2OH?
A) Functional
B) Symmetrical
C) Configurational
D) Conformational
Solution
As we know that in organic chemistry isomers play a major role. In reaction time isomer plays an important role. Depending on the isomer of reactant only product will be obtained. In some cases equal amounts of two isomers will be obtained in the reaction. Acids and esters are called Tautomer. Alcohol and ethers are also called Tautomer. The tautomer isomers are commonly called functional isomers.
Complete answer:
The given molecular formula are CH3CH2OCH3 and CH3CH2CH2OH.
The molecular formula methyl ethyl ether is CH3CH2OCH3.
The molecular formula propanol is CH3CH2OCH3.
We can draw the structural formula methyl ethyl ether as,
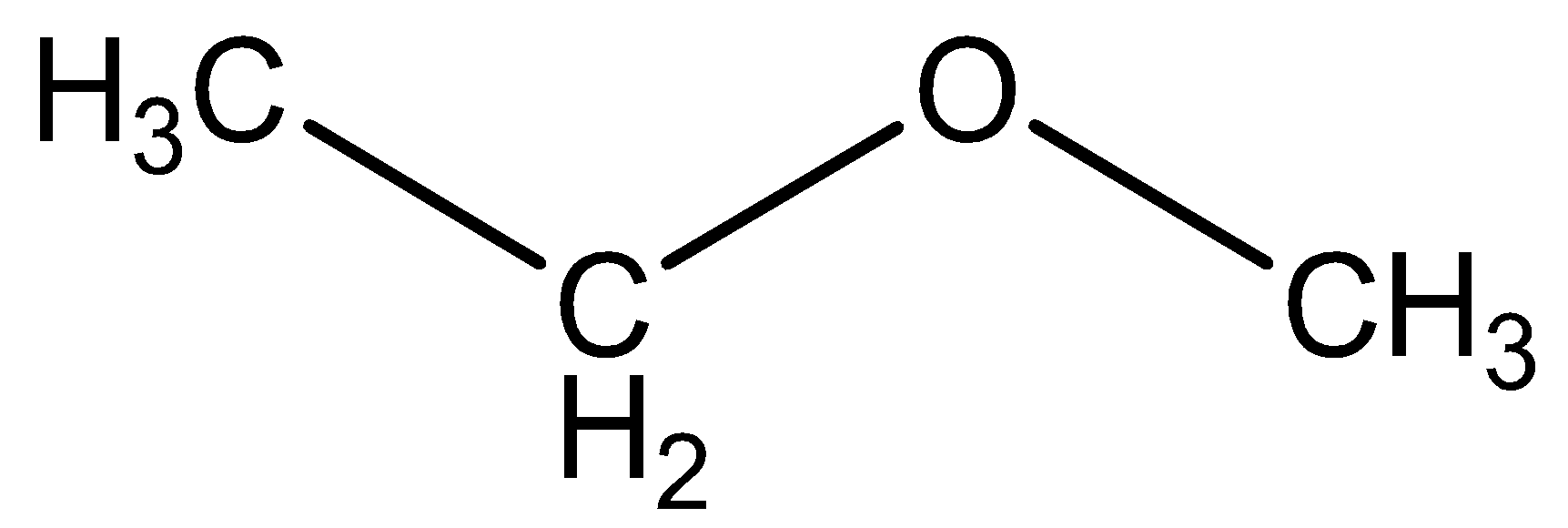
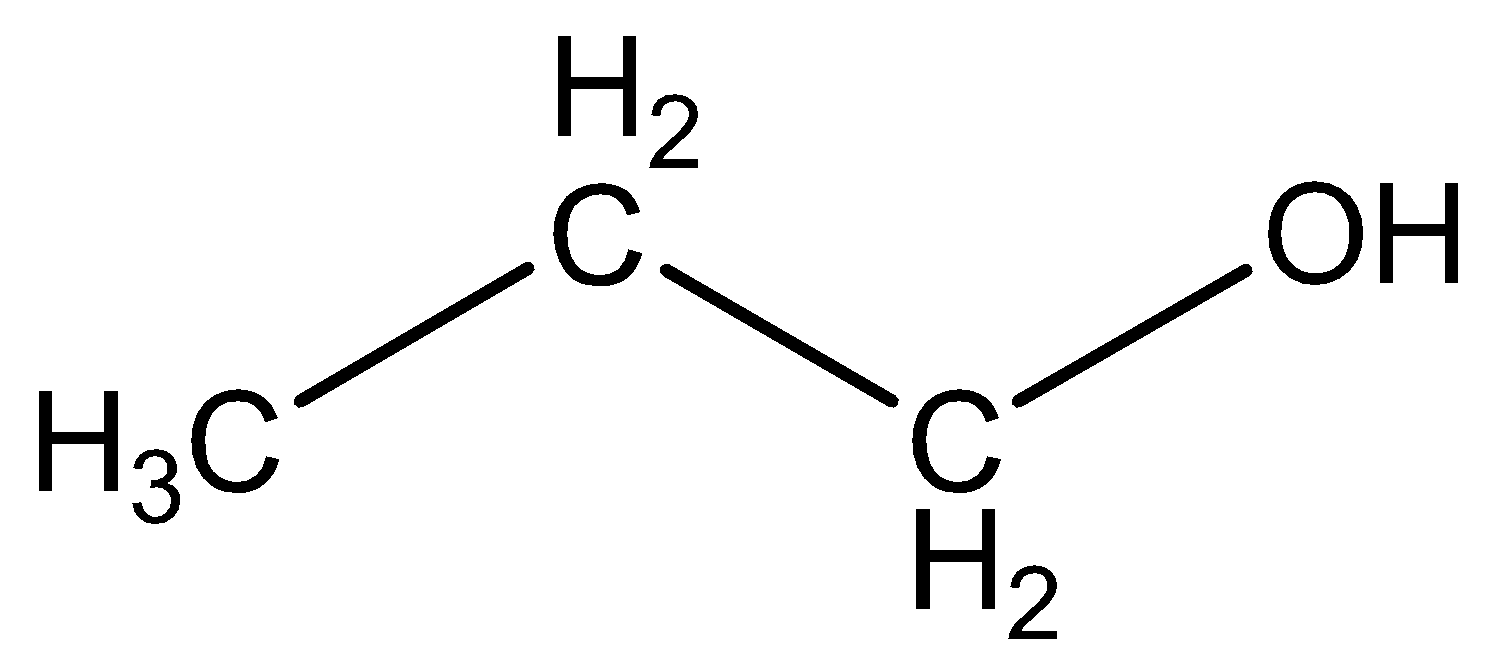
We can draw the structural formula propanol as,
The functional group in methyl ethyl ether CH3CH2OCH3 is ether.
The functional group in propanol CH3CH2OCH3 is alcohol.
The alcohol and ether are the functional isomers. So, CH3CH2OCH3 and CH3CH2CH2OH are the functional isomers.
According to the above discussion, we conclude CH3CH2OCH3 and CH3CH2CH2OH are the functional isomers.
Hence, option A is the correct answer.
Note:
We need to remember that the isomer of the organic molecule is divided by two types. There are constitutional isomers and stereoisomers. Further constitutional isomers are classified as chain isomer, position isomer, functional isomer, Metamers, Tautomer and ring chain isomer. The stereoisomers are classified as geometrical isomer and optical isomer. The isomer means the same molecular formula but different in structural or positional or functional or chain of the carbon atom in the organic molecule. Optical isomer should have a chiral carbon or asymmetric centre in the molecule. In organic chemistry isomers play a major role. In reaction time isomer plays an important role. Depending on the isomer of reactant only product will be obtained. In some cases equal amounts of two isomers will be obtained in the reaction.
