Question
Question: What type of indicator is phenolphthalein? A. Acidic B. Basic C. Equivalent (titration) D. N...
What type of indicator is phenolphthalein?
A. Acidic
B. Basic
C. Equivalent (titration)
D. Neutral
Solution
Try to recall that an indicator is something which shows that a change has happened and is used to check whether a substance is acid or base. Phenolphthalein is a chemical compound with chemical formula C20H14O4 and is used as an indicator in acid-base titration. Now, by using this you can easily find the correct option from the given options.
Complete step by step answer:
- It is known to you that phenolphthalein is an acid base indicator used to test the pH of the solution.
- Phenolphthalein is an organic compound with chemical formula C20H14O4 and is a synthetic indicator.
- It is usually prepared by reacting phthalic anhydride and two molecules of phenol in an acidic medium.
- Phenolphthalein (HIn) is weakly acidic in nature and in aqueous solution, it dissociates into H+ and In− ions. Here, the H denotes the hydrogen protons and the In indicates the indicator part of the ion.
- The pink colour of the solution is due to the concentration of In− ions in the solution.
- Under acidic conditions, the concentration of In− in the solution is very low and concentration of H+is high, hence it is colourless.
- Similarly, under basic conditions, the concentration of H+ ions is very low and concentration of In− is high, hence the solution is pink coloured.
- The structure of phenolphthalein is as follows:
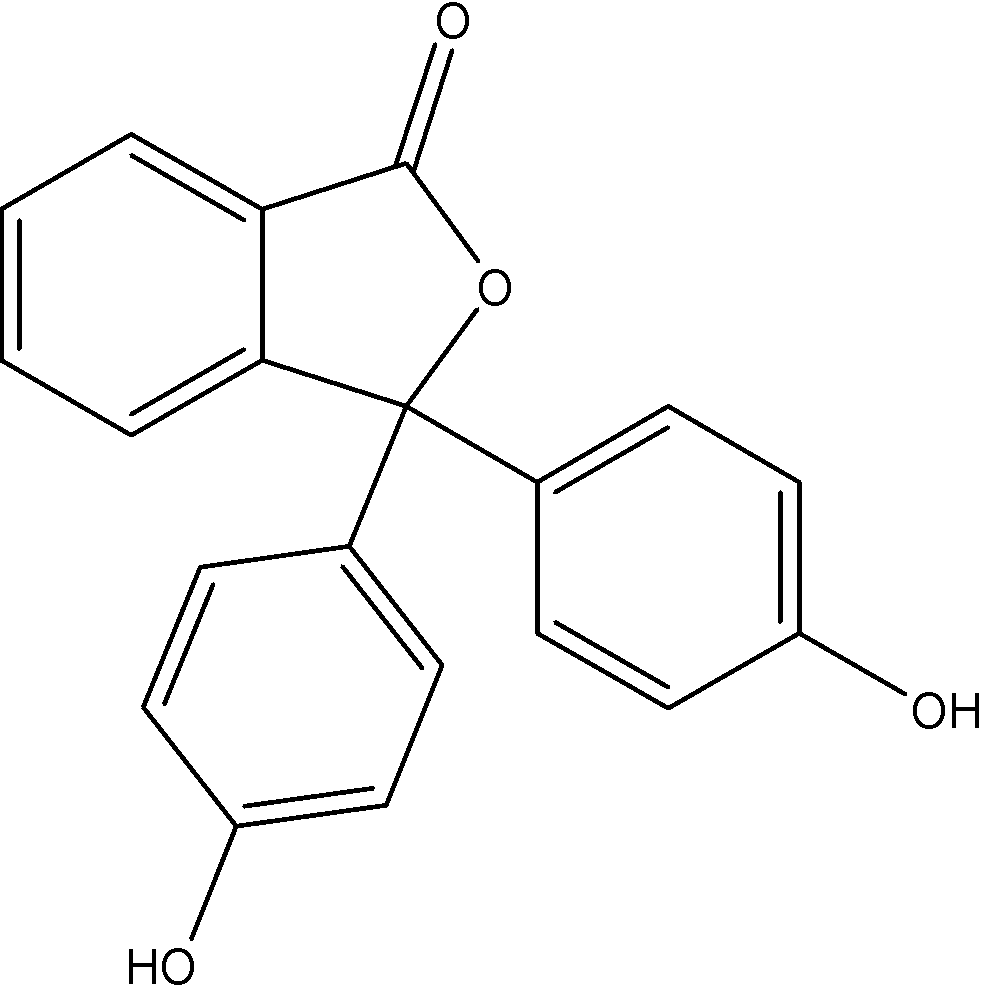
So, the correct answer is “Option A”.
Note: - It should be remembered to you that the phenolphthalein indicator is a weak colourless acid that forms pink anions when it is dissolved in water. Although the anions are pink, the solution remains colourless in the presence of an acid.
- If the pH of the solution is 8.2 or above, then the number of anions increases, causing the solution to pink, the anions of the phenolphthalein molecule actually act as an indicator.
