Question
Question: What stereoisomers are obtained from hydroboration-oxidation of \( 1 \) -ethyl cyclohexene?...
What stereoisomers are obtained from hydroboration-oxidation of 1 -ethyl cyclohexene?
Solution
1 -ethyl cyclohexene is a cycloalkene in which ethyl group is substituted at the C1 carbon. Hydroboration-oxidation reaction is the addition of boron trihydride to an alkene further the addition of hydroxide replaces the BH2 group and substitutes the hydroxyl group. Different isomers were obtained when hydroboration-oxidation of 1 -ethyl cyclohexene is done.
Complete answer:
Stereoisomers are the chemical compounds that contain the same molecular formula, but there is a difference of direction of arrangement of atoms or groups in the molecule.
1 -ethyl cyclohexene is a cycloalkene in which an ethyl group is substituted at the C1 carbon. Hydroboration is the reaction of addition of simple boranes like BH3 to alkene, which results in the addition of H and BH2 units leading to the formation of alkyl boranes.
Later this alkyl boranes when treated with hydrogen peroxide which is an oxidation reaction leads to the formation of alcohols as BH2 is replaced by hydroxide ion from hydrogen peroxide. There is no formation of intermediates as only the transition state was formed.
The hydroboration-oxidation of 1 -ethyl cyclohexene is:
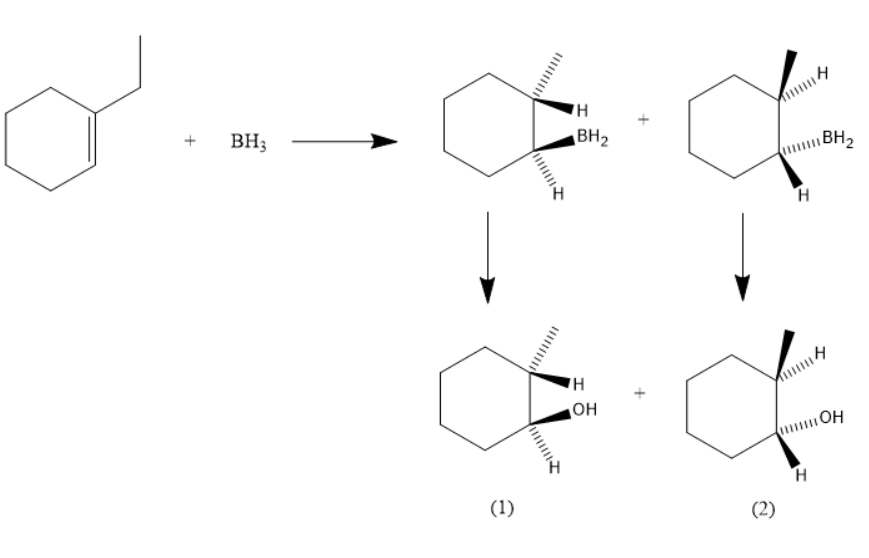
Though the formed two products are with the same molecular formula, there is a difference in the arrangement of groups that leads to the formation of stereoisomers.
The product 1 is (1S,2R)−2−ethylcyclohexanol
The product 1 is (1R,2S)−2−ethylcyclohexanol .
Note:
The overall reaction favours the anti-markovnikov's rule as the hydroxide group is attached to the carbon containing more number of hydrogen atoms. The addition of borane favours the syn-addition as both H and BH2 units were added at the same side of the double bond.
