Question
Question: What starting material is needed to synthesize the following compounds? 
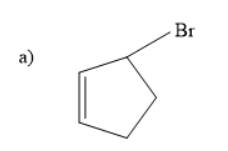
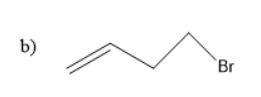
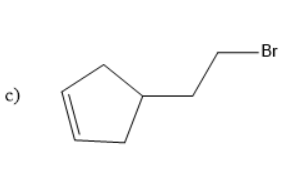
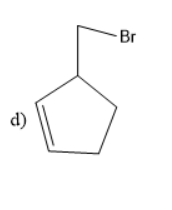
Solution
Alkenes are unsaturated compounds consisting of double bonds and when alkenes treated with potassium permanganate, diols were formed. The potassium permanganate is an oxidizing agent. The diols formed when treated with sodium hydride, a base then the above products will be formed.
Complete answer:
Alkenes are unsaturated compounds due to the presence of double bonds. Alkenes when treated with potassium permanganate undergoes an additional reaction. The diols will be formed. Diol is a compound with two hydroxyl groups in the molecule. When this diol is treated with sodium hydride and dimethyl formamide, cyclisation occurs and oxygen atom goes into the ring and thus the compound formed has the hydroxyl substituent and cyclo butoxy compound.
Potassium permanganate is an oxidizing agent and sodium hydroxide is a strong base. Both these compounds are used for the diol’s formation. The sodium hydride belongs to the hydride family, a strong base and dimethylformamide can be used as a solvent.
The reaction will be as follows:
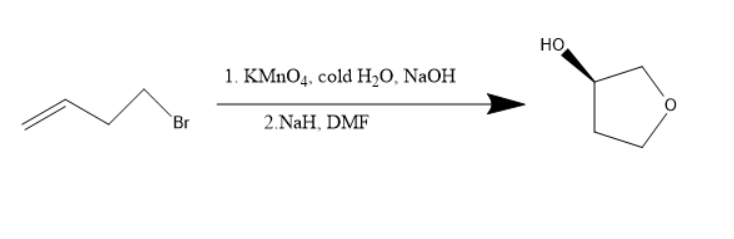
Thus, the starting material is an alkene with bromine as substituent.
Thus, 1-bromo but- 4-ene is the starting material.
So, the correct answer is “Option b”.
Note:
Alkenes are also known as olefins. Due to the presence of double bond, alkenes undergo addition reactions; the addition of potassium permanganate in presence of a base is an addition reaction to form diols. The diols further treatment with sodium hydride forms a cyclo alkoxy compound.
