Question
Question: What sort of hybridization does the central atom in \[S{F_4}\] have?...
What sort of hybridization does the central atom in SF4 have?
Solution
Atoms, the smallest unit into which matter is often divided without the discharge of electrically charged particles. It is also the smallest unit of matter that has the characteristic properties of an element. As such, the atom is the basic building block of chemistry.
Complete Answer:
The molecule will have a complement of 34 valence electrons, 6 from sulphur, and seven from each of the four fluorine atoms. In Sulphur tetrafluoride, the central atom of SF4 is sp3d hybridized. Sulphur forms 4 single bonds and has 1 lone pair, which suggests that its steric number, which is that the name given to the number of regions of electron density, is adequate to 5 .
The SF4 Lewis structure is the combination of 34 electron and 5 electron pairs around the Sulphur, where there are 4 bonding pairs and 1 lone pair. This electron arrangement is named 'Trigonal Bipyramidal'. The rationale behind this is often that the lone pair prefers one among the equatorial positions.
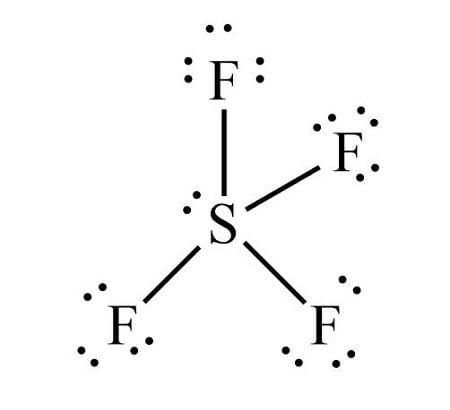
The example of the see-saw shape of the molecule is the sulfur tetrafluoride, or SF4 . Sulfur is the central atom, two fluorine atoms are on the equatorial plane, and two are on the axial plane.
Note:
Trigonal bipyramidal (sp3d) is the shape of SF4 with one equatorial position occupied by 1 lone pair. It's a see-saw shape because it contains four bond pairs and one lone pair. The equatorial F atoms are 120 from one another, therefore the axial or equatorial bond angle is 90∘ .
