Question
Question: What should be the correct IUPAC name for diethyl bromomethane? A: 1-Bromo-1,1-diethylmethane B:...
What should be the correct IUPAC name for diethyl bromomethane?
A: 1-Bromo-1,1-diethylmethane
B: 3-Bromopentane
C: 2-Bromopentane
D: 1-Bromopentane
Solution
Hint : IUPAC stands for International Union of Pure and Applied Chemistry. It is the world authority on the chemical nomenclature as well as terminology. Thus, IUPAC nomenclature is basically a method of naming organic compounds according to certain rules.
Complete Step By Step Answer:
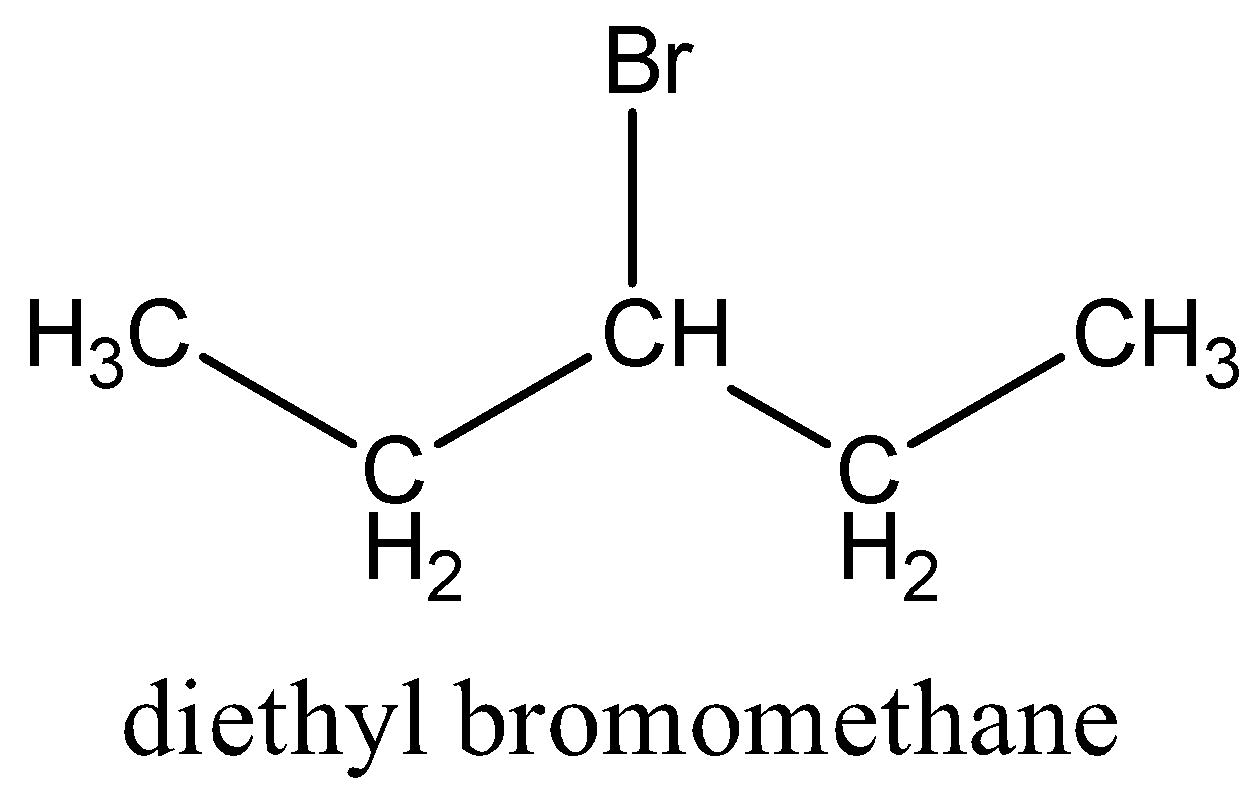
IUPAC provides consistency to the names of organic compounds. It enables every compound to possess a unique name, which otherwise is not plausible with the common names. The chemical structure for diethyl bromomethane is shown below:
Now, let us have a look at the important rules that need to be followed during naming of a compound:
To determine the suffix of the compound name, recognise the functional group present in the compound (as listed in table).
| Functional group | Suffix |
|---|---|
| Alkane | -ane |
| alkene | -ene |
| Alkyne | -yne |
| Alcohol | -ol |
| Aldehyde | -al |
| Ketone | -one |
| Carboxylic acid | -oic acid |
| ester | -oate |
The given compound i.e. diethyl bromomethane is an alkane so suffix in our case will be –ane.
To determine the prefix of the name of compound, identify the longest and continuous chain of carbon containing the functional group and count the number of carbon atoms in the chain as shown in table below.
| Number of carbon atoms | Prefix |
|---|---|
| 1 | Meth- |
| 2 | Eth- |
| 3 | Prop- |
| 4 | But- |
| 5 | Pent- |
| 6 | Hex- |
| 7 | Hept- |
| 8 | Oct- |
| 9 | Non- |
| 10 | Dec- |
In the given compound i.e. diethyl bromomethane, the number of carbon atoms in the longest chain is 5. That means the prefix in our case is pent-.
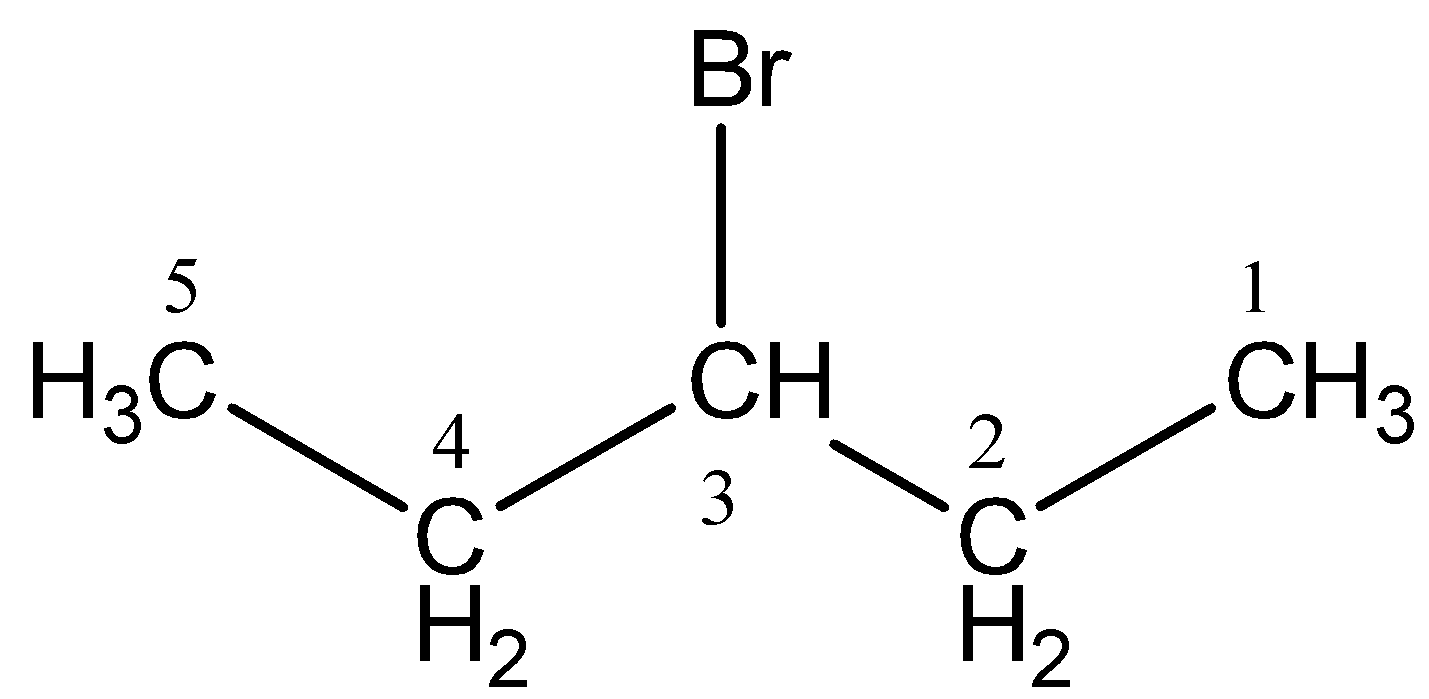
Numbering of the carbons in the longest chain of carbon (Remember that If the organic molecule is not an alkane (and has a functional group) you have to initiate numbering such that the functional group is placed on the carbon having the lowest possible number). The halogen present acts as a substituent on the carbon chain. The halogens can be represented as follows:
| F | Fluoro- |
|---|---|
| Br | Bromo- |
| Cl | Chloro- |
| I | Iodo- |
In the given compound, we can number as shown below:
Check if there are any branched groups, name them and allocate the number of the carbon atom to which that group is linked. The branched groups must be mentioned before the name of the main chain in an alphabetical order.
In the given compound, the halide (bromine) group present on the third carbon will be treated like a branched group. It will be written as 3-bromo- before the main chain name.
Finally, combine the elements of the compound name in the specified order as: branched groups, prefix, suffix according to the functional group and its location along the longest carbon chain.
Therefore, the given compound will have IUPAC name as: 3-bromopentane. Hence, the correct answer is Option B.
Note :
IUPAC nomenclature creates a standardized method to name the chemical compounds. Common nomenclature employs the older names for organic compounds rather than using the prefixes for the carbon chain. IUPAC nomenclature even provides certain rules for the naming of ions as well.
