Question
Question: What role does the molecular interaction play in a solution of alcohol and water ?...
What role does the molecular interaction play in a solution of alcohol and water ?
Solution
Interaction between two or more molecules of Alcohol and two or more molecules of water have hydrogen bonding between them. In the same way solutions of water and alcohol too have hydrogen bonding between them.
Complete step by step answer:
- Hydrogen bonding in H2O is represented as
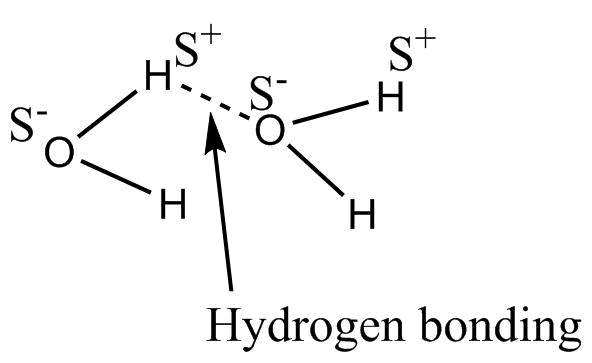
An intermolecular hydrogen bonding is seen in between water molecules this H- bonding is strong bonding.
-Hydrogen bonding in alcohol is represented as :-
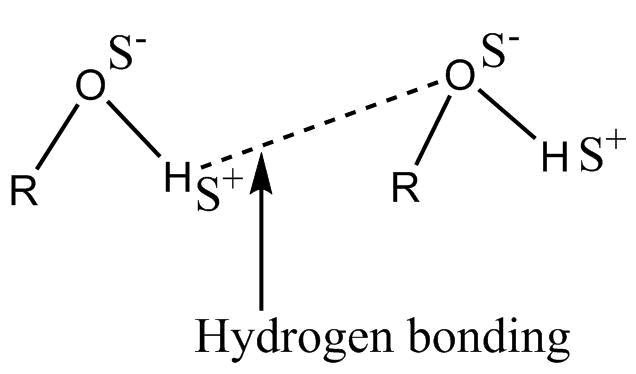
This is also a strong intermolecular bond.
-When alcohol is mixed in water, a solution is formed represented as :-
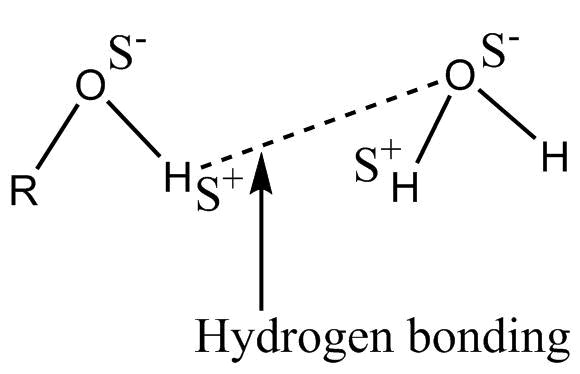
This intermolecular H – bonding is weaker as compared to Alcohol – Alcohol or water-water H bond
Moreover, the size of alcohol also has a deciding factor in molecular interactions.
As the molecular weight of the alkyl chain of alcohol becomes less soluble is water because large alkyl groups prevent formation of hydrogen bonds.
Additional Information: Hydrogen bonding is a type of intermolecular interaction in which hydrogen is located between atoms which have strong affinity of electrons. More electronegative elements such as fluorine, Oxygen, Nitrogen shows hydrogen bonding to a greater extent.
Note: Also, the solution mixture of alcohol and water is a non-ideal solution. This solution shows a positive deviation and thus, its vapor pressure is increased. Due to increased vapour pressure, the boiling point of the solution becomes lower.
The solution of alcohol and water is a homogeneous solution that can no longer be distinguished from each other.
