Question
Question: What reagent is used in the Hinsberg test of amines? (a) \(({ CH }_{ 3 }CO{ ) }_{ 2 }O\) and pyrid...
What reagent is used in the Hinsberg test of amines?
(a) (CH3CO)2O and pyridine
(b) C6H5SO2Cl in aq. NaOH
(c) NaNO2 in aq. NaNO2
(d) CH3 (excess) followed by AgOH
Solution
Hint: In order to distinguish between the primary, secondary and the tertiary amines we use Hinsberg’s reagent. Hinsberg’s reagent is prepared by the chlorination of benzene sulphonic acid by using phosphorus oxychloride. Any other suitable chlorinating agent can also be used.
Complete step by step solution:
Hinsberg’s reagent is used in order to distinguish between primary, secondary and tertiary amines. Let us first understand these three types of amines:
In primary amines or 1o amines two sp3 hybrid orbitals of Nitrogen overlap with the 1s orbital of Hydrogen atoms, one sp3 orbital contains a lone pair while the remaining sp3 orbital overlaps with the sp3 orbital of a carbon atom belonging to an alkyl group or with a sp2 orbital of a carbon atom belonging to an aryl group.
In secondary or 2o amines one sp3 hybrid orbital of Nitrogen overlaps with the 1s orbital of a Hydrogen atom, one sp3 orbital contains a lone pair while the remaining two sp3 orbitals overlap with the sp3 orbitals of carbon atoms of two alkyl groups or with sp2 orbitals of carbon atoms of two aryl groups.
In tertiary or 3o amines one sp3 orbital of Nitrogen atom contains a lone pair while the remaining three sp3 orbitals overlap with the sp3 orbitals of carbon atoms of three alkyl groups or with sp2 orbitals of carbon atoms of three aryl groups.
In order to distinguish between them, Hinsberg’s reagent (Benzenesulfonyl chloride) is used.
A primary amine reacts with the Hinsberg’s reagent in the presence of aq. KOH solution to give a clear solution. When this solution is acidified, an insoluble N-alkyl benzene sulphonamide is produced. The reaction is given below:
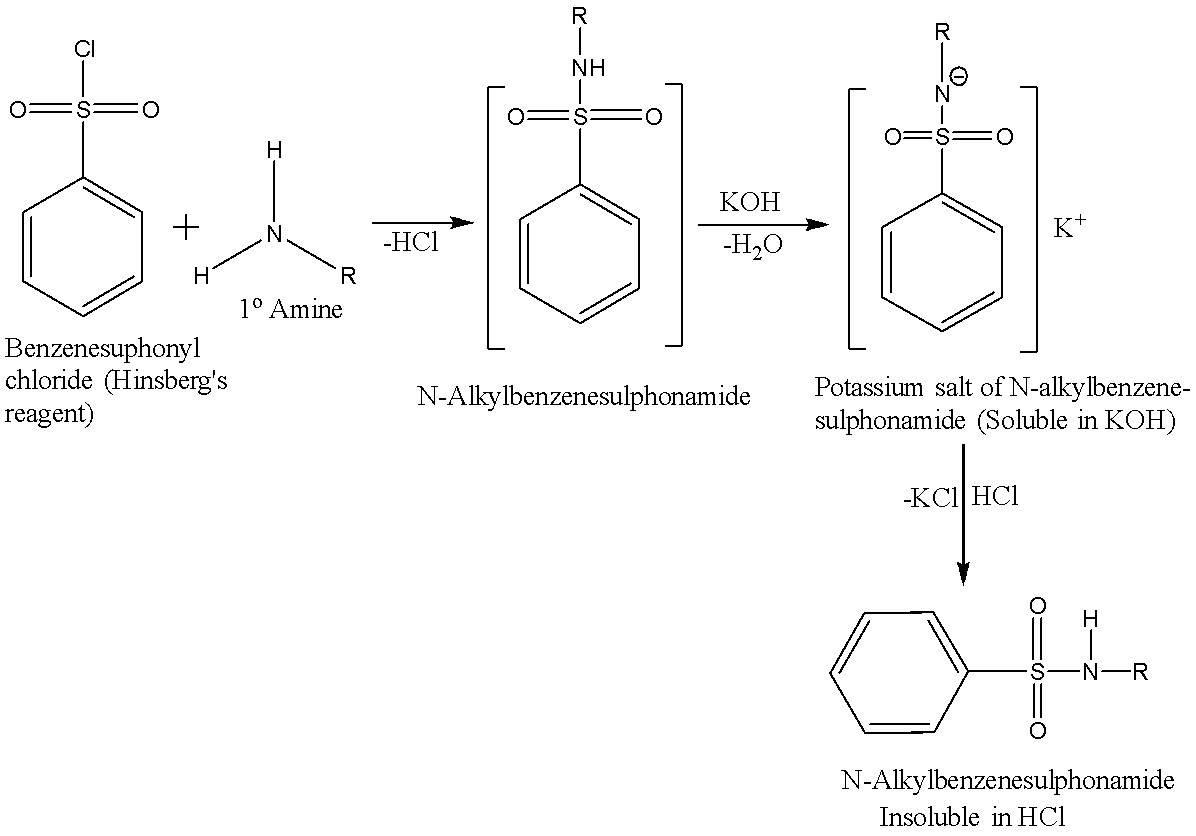
A secondary amine reacts with the Hinsberg’s reagent in the presence of aq. KOH solution to give an insoluble N,N-dialkyl benzenesulfonamide and there is no change observed when acid is added to it. The reaction is given below:
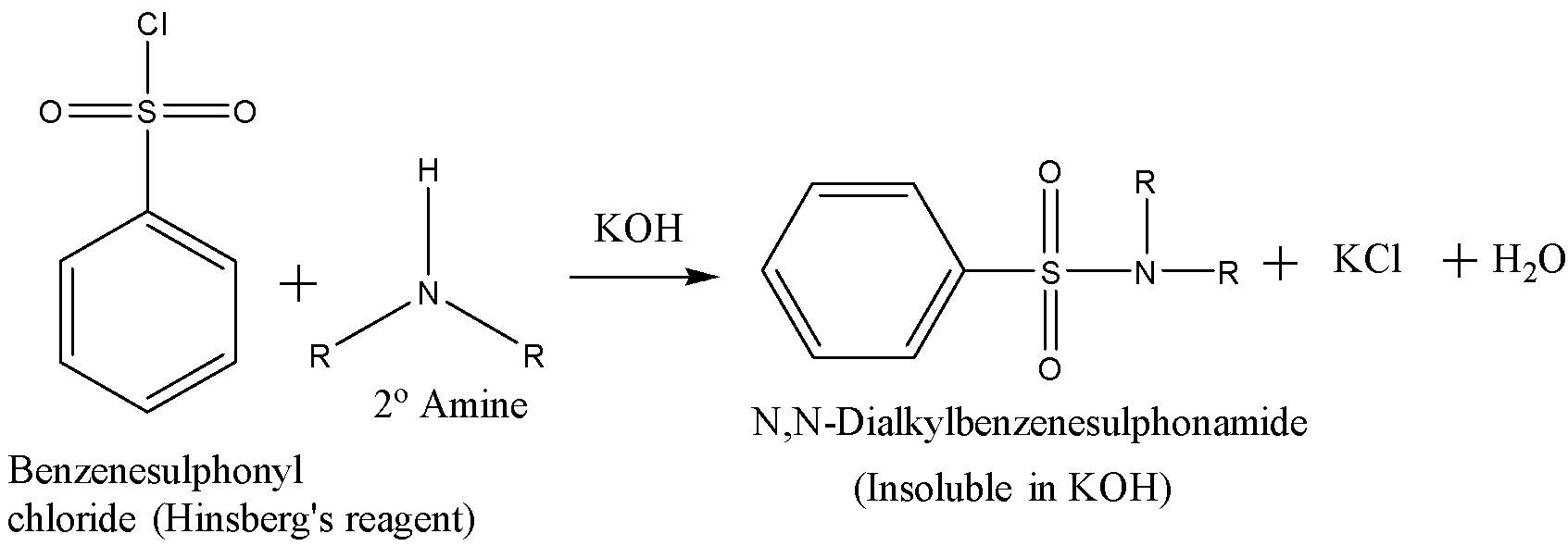
A tertiary amine does not react with Hinsberg’s reagent and remains insoluble in the alkaline solution. On acidification of the solution a clear solution is obtained because of the formation of the ammonium salt. The reaction is given below:
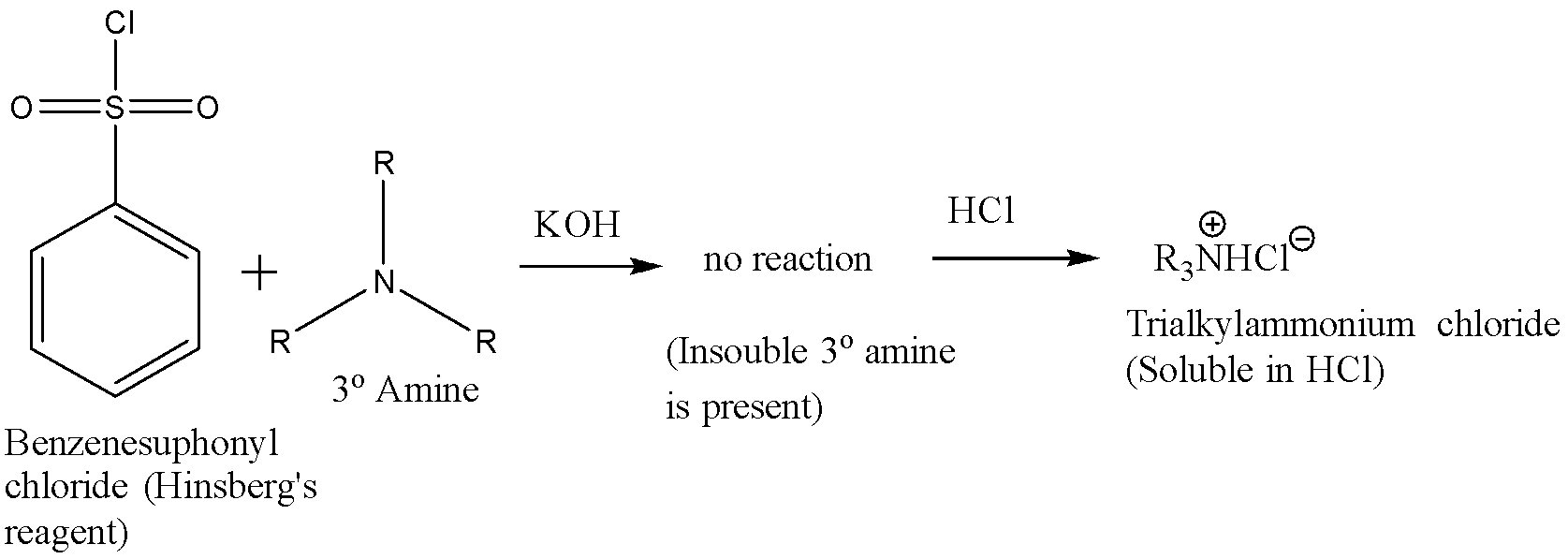
Hence the correct answer is (b) C6H5SO2Cl in aq. NaOH.
Note: For providing the alkaline medium, both NaOH and KOH can be used with Hinsberg’s reagent. A primary amine forms a potassium salt of N-Alkyl Benzene Sulphonamide when it reacts with the Hinsberg’s reagent in the presence of aq. KOH since a primary amine has an acidic hydrogen on the N-atom of the amine group.
