Question
Question: What product will be formed? 
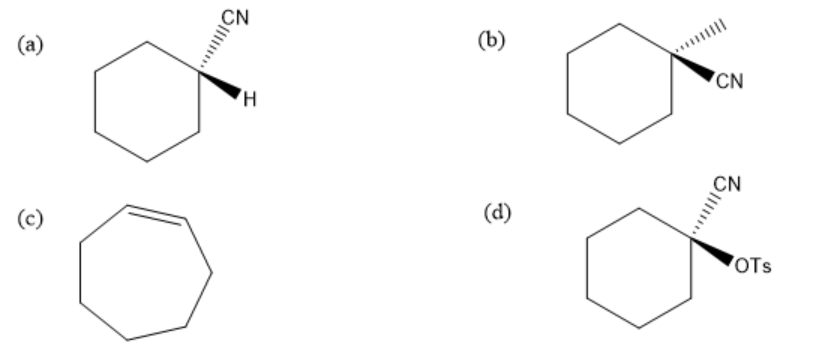
Solution
Hint : There are many reagents in organic chemistry for different chemical reactions. Hg(OAc)2 and NaBH4,OH− when used together they introduce −OH group on sp2 carbon making it saturated sp3 . TsCl when attack on the −OH group converts it into −OTs which is a very good leaving group and KCN in the above reaction is used to introduce the −CN group.
Complete Step By Step Answer:
Reagents are chemical substances which are used in numerous organic reactions to synthesize desired products.
The reaction given is:
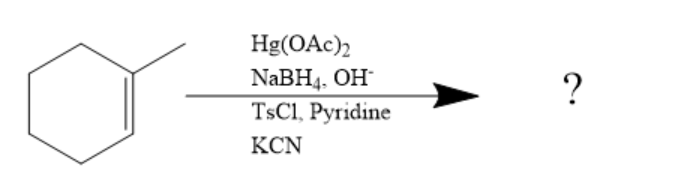
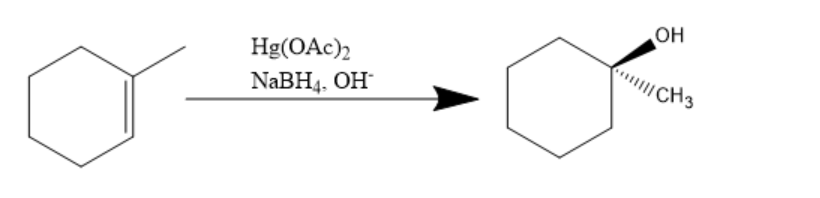
In the above given reaction, first Hg(OAc)2 and NaBH4,OH− is added and it will introduce −OH group on sp2 carbon making it saturated sp3 according to markovnikov's rule.
It states that with the addition of HX or other polar reagent to an asymmetric alkene, the hydrogen gets attached to the carbon with more number of hydrogen substituents, and the X group or electronegative part gets attached to the carbon with more number of alkyl substituents.
Hence, the reaction proceeds as:
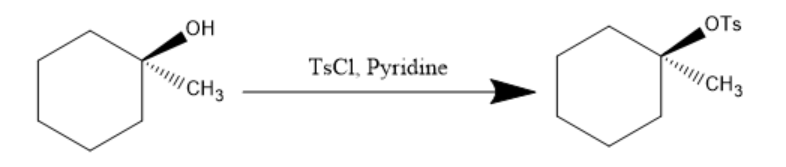
Now, pyridine (being a base) abstract the hydrogen from hydroxyl group and TsCl is added, it attack on −OH group converts it into −OTs which is a very good leaving group and the reaction proceeds as follow:
Now, the KCN is added to the product and −OTs being a very good leaving group, leaves and CN− attacks and is introduced to the compound. The reaction can be written as:
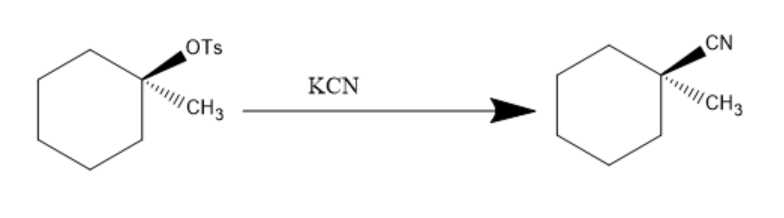
Hence, the final product of above given reaction is:
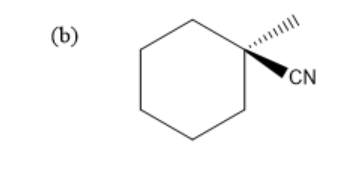
Therefore, the correct option is (b).
Note :
For the addition of hydroxyl group to a double bond (unsaturated bond) through the markovnikov rule, we use reagents as BH3,THF,H2O2 and OH−. In this reaction, the hydrogen gets attached to the carbon with more alkyl substituents, and the X group or electronegative part gets attached to the carbon with more hydrogen substituents.
