Question
Question: What product is formed when acetic acid reacts with \({{P}_{2}}{{O}_{5}}\) ? A. Acetyl chloride ...
What product is formed when acetic acid reacts with P2O5 ?
A. Acetyl chloride
B. Trichloro acetic acid
C. Acetic anhydride
D. Dichloro acetic acid
Solution
P2O5 called phosphorus pentoxide. It is a good dehydrating agent means it removes water very easily and the reaction is called dehydration reaction. The molecular formula of acetic acid isCH3COOH .
Complete Solution :
- In the question it is given what product we will get when acetic acid reacts with phosphorus pentoxide.
- The reaction of acetic acid with phosphorus pentoxide is as follows.
2CH3COOH+P2O5→(CH3CO)2O+H2O
- In the above reaction two moles of acetic acid react with one mole of phosphorus pentoxide and form one mole of acetic anhydride and one mole of water as the products.
- Therefore acetic anhydride is going to form when acetic acid reacts with P2O5 .
- The structure of the acetic anhydride is as follows:
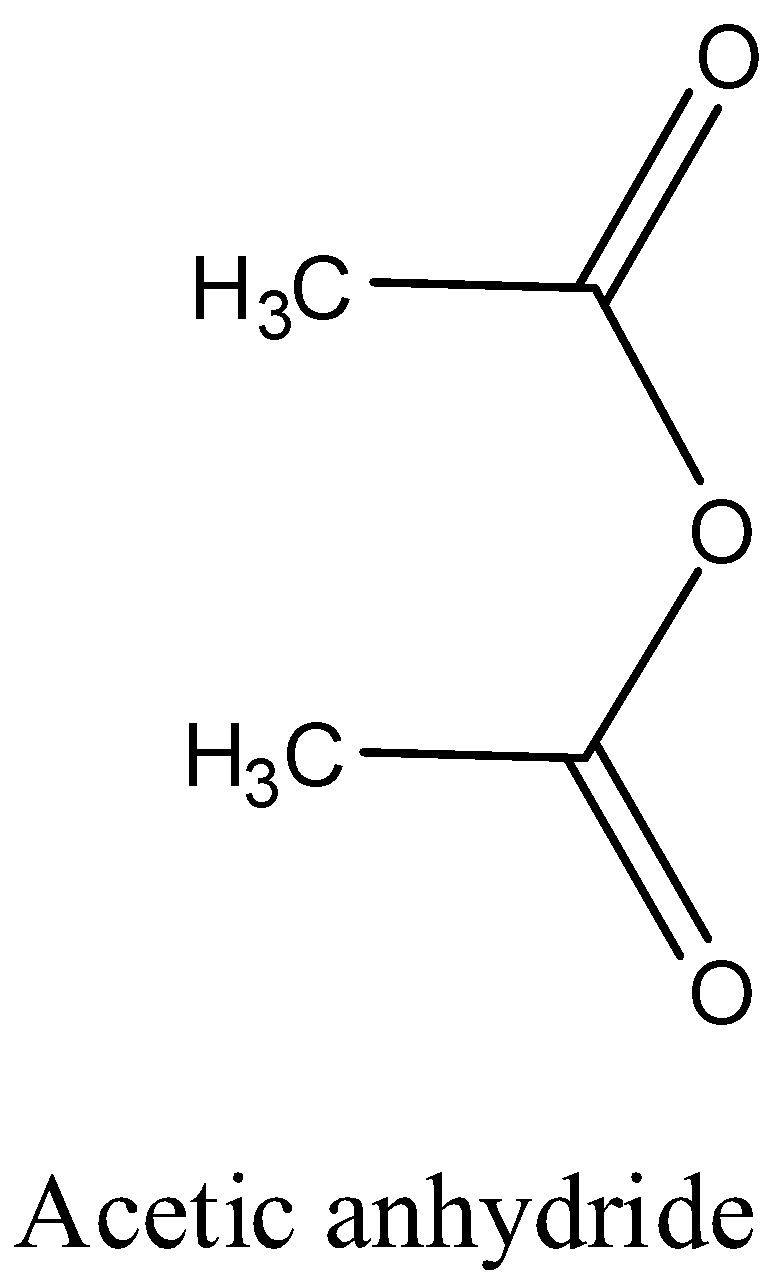
So, the correct answer is “Option A”.
Additional Information:
- The molecular formula of acetic anhydride is C4H6O3 .
- Acetic acid also called as ethanoic acid.
- Acetic anhydride is commonly written as Ac2O .
- Acetic anhydride is generally used in organic synthesis as an acetylating agent.
- In the preparation of aspirin acetic anhydride plays a crucial role.
Note: When we are going to add the acetic anhydride to the water again acetic acid is going to form and we can see the chemical reaction of acetic anhydride with water as follows:
(CH3CO)2O+H2O→2CH3COOH
One mole of acetic anhydride produces two moles of acetic acid and the above reaction is called hydration reaction.
