Question
Question: What product is formed in the treatment of cyclopentene with the bromine water? (A) trans-3-bromoc...
What product is formed in the treatment of cyclopentene with the bromine water?
(A) trans-3-bromocyclopentanol
(B) cis-2-bromocyclopentanol
(C) trans-2-bromocyclopentanol
(D) cis-3-bromocyclopentanol
Solution
In order to answer the question, we must have an idea about different organic reactions that will be taking place. So, it will become easier to answer what will be the product formed when cyclopentene is treated with the bromine water. This reaction will be following Markovnikov’s rule.
Complete Solution :
The treatment of the cyclopentene with the bromine water is an electrophilic substitution reaction.
In the first step when the Bromine molecule and the water molecule reacts with each other, it will lead to the formation of the Bromohydrins. The reaction of the formation of the Bromohydrin is given below:
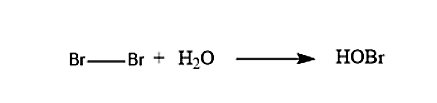
- The bromohydrin will break in such a way that the Halide is the negative species, i.e. Br−, while the hydrin is the positive species i.e. HO+
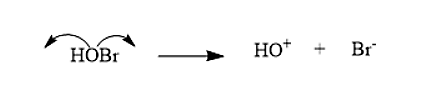
- The cyclopentene will react with the formed bromohydrin to give Trans-2-bromopentanol. The and will attack across the double bond. This reaction will be following Markovnikov’s rule. Formation of the Trans-2-bromopentanol is given below:
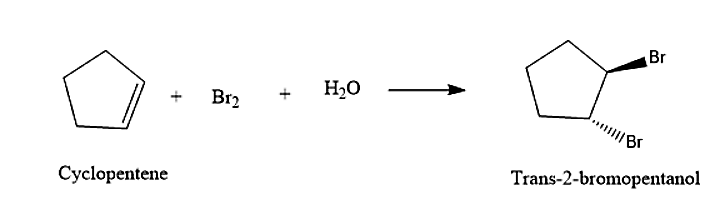
So, the correct answer is “Option C”.
Note: - Bromine water is usually used to test whether the given compound is saturated or unsaturated.
- Organic compounds such as alkene, phenols and aniline will undergo reaction with bromine water readily.
- When the colour of the bromine water changes, it will indicate the presence of an unsaturated compound.
