Question
Question: What other reaction is commonly used as a functional group test for alkenes?...
What other reaction is commonly used as a functional group test for alkenes?
Solution
For answering this question, we have to use the two tests which are:
-Bromine decolourization test
-Baeyer’s unsaturation test
Complete answer:
We will discuss both the tests one by one which are the bromine decolourization test and Baeyer unsaturation tests.
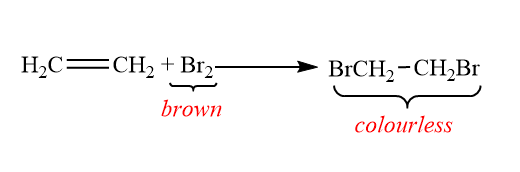
Test-1: Bromine decolourization test:
This test is a qualitative test. The bromine test is used for determination of the presence of unsaturated carbon, phenols and anilines. In this test a sample is treated with a small amount of bromine in an organic solvent, such as dichloromethane or carbon tetrachloride. When the bromine reacts with unsaturated carbon or phenol or aniline in the sample, the deep brown colour of bromine disappears. So when the alkene in the sample reacted with bromine, the deep brown colour disappeared.
So on adding a few drops of the Br2 in the CCl4 , the alkene will decolourize the bromine solution.
When alkene reacts with Bromine:
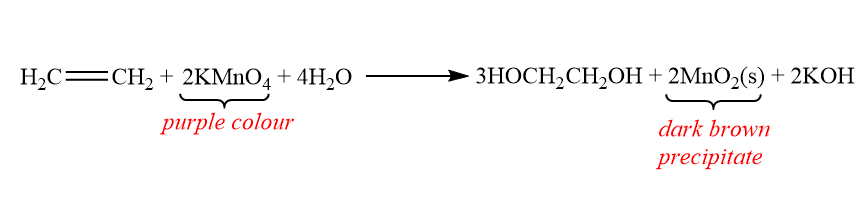
Test-2: Baeyer unsaturation test:
The Baeyer unsaturation test for unsaturated compounds in which potassium permanganate is used. This is used for the presence of carbon-carbon double bonded compound that is alkene or carbon-carbon triple bonded compound that is alkyne.
For this test we use dilute Potassium permanganate which is for oxidizing the carbon-carbon double bond or triple bond. It is an oxidation reaction because the double bond is replaced by the hydroxyl group, which is an OH group. The oxidation number of carbon goes from +1 to +2, that means it loses an electron. The alkene will give dark brown precipitate.
The reaction is shown below:
Note:
In the bromine test, bromine solution is added to the unsaturated hydrocarbon, the brown colour disappears if the given hydrocarbon is unsaturated. As the Bromine forms an additional product with the unsaturated hydrocarbon.
