Question
Question: What molecular structure is expected for iodine molecules according to the VSEPR model? A.T-shaped...
What molecular structure is expected for iodine molecules according to the VSEPR model?
A.T-shaped
B. See-saw
C. Trigonal planar
D. Linear
Solution
We know that valence shell electron pair repulsion (VSEPR) theory is used to determine the geometry of molecules from the electron pairs surrounding the central atom.
Complete step by step answer:
First, we draw the structure of ICl3 molecule. We know that iodine is an element belonging to group 17 of the periodic table. Chlorine is also an element that belongs to the same group as iodine. The number of valence electrons of iodine is 7. Out of seven valence electrons, three electrons are shared with three chlorine atoms to form three I−Cl bond and two pairs of lone pair electrons. So, the structure of ICl3 molecule is,
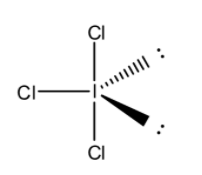
From the above Lewis structure of ICl3 molecule, we clearly see that the position of two lone pairs at the equatorial position in the trigonal bipyramidal geometry. The positions of lone pairs are120∘ apart, so they experience least repulsion compared to axial position. So, the shape of ICl3 molecule is T-shaped.
So, the correct answer is “Option A”.
Additional Information:
According to VSEPR theory, lone pair electrons repel each other more strongly than that of bond pair electrons. So, the decreasing order of repulsion is lp-lp>lp-bp>bp-bp.
So, repulsion between bond pair-bond pair is least and between lone pair-lone pair is highest.
Note:
Students might think that as Iodine has three bond pairs in ICl3, its molecular shape is trigonal planar. But it is not correct. The two lone pairs also impact on the shape of ICl3 molecule. If only three bond pairs present in a compound, then its molecular shape is trigonal planar, such as, in case of BH3.
